Application Note: Sputter Reduction of Iron Oxide
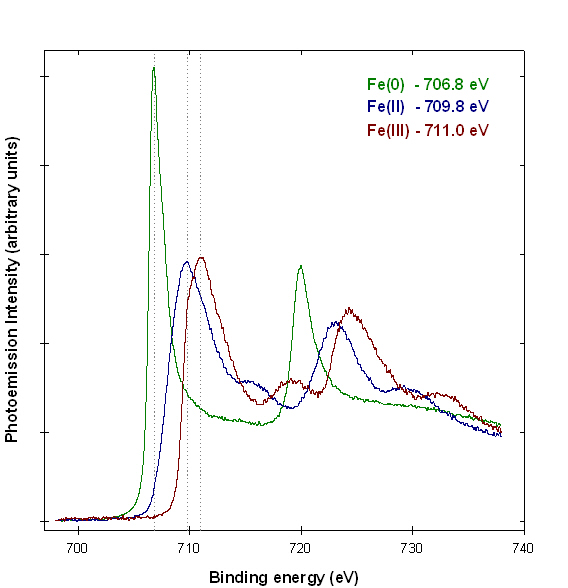
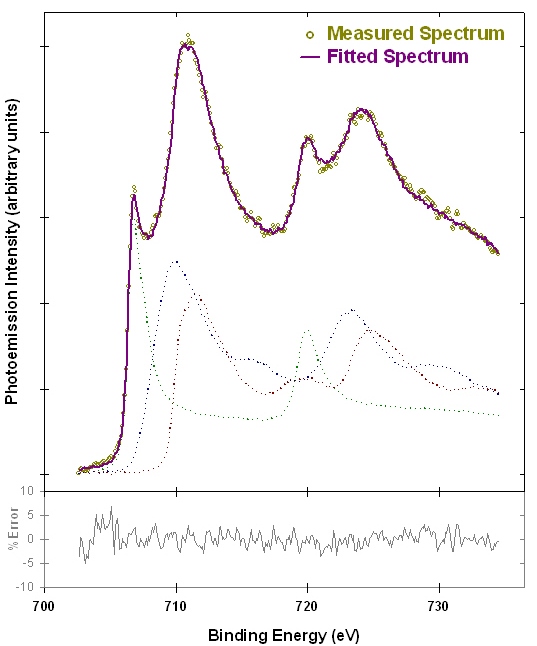
X-ray photoelectron spectroscopy (XPS ; also known as ESCA – electron spectroscopy for chemical analysis) is a surface analysis technique that can be used to distinguish the oxidation state of transition metals(1). Figure 1 shows examples of iron XPS spectra from reference compounds. The spectra exhibit a variety of features that allow the iron metal, iron (II) and iron (III) species to be distinguished. Figure 2 shows an example linear peak fit of a mixed iron oxidation state sample. The measured spectrum is well fit by a linear combination of the reference spectra from Figure 1, and indicates that the analyzed area contains 28±2% Fe(0), 41±5% Fe(II), and 32±6% Fe(III).
The study of transition metal oxidation states on solids surfaces provides information on many important industrial processes, including corrosion, catalysis and lubrication. However, in many cases, the outermost surface of a sample may be altered or contaminated, and needs to be cleaned to expose pristine material for analysis. Cleaning in situ in a vacuum chamber can be performed by using an ion beam to etch material from the surface of a solid sample. This process is known as ion beam sputtering. Using an ion beam to sequentially etch material from a surface during analysis can also produce a depth profile, which shows the distribution of elements from the surface into the bulk of a sample. Depth profiling is used extensively for analysis of layered samples. However, using an ion beam for cleaning or profiling can change the composition of a material through effects such as preferential sputtering and sputter reduction (2,3). In a material with multiple elements, elements may sputter at different rates, and hence change the stoichiometry of the analyzed surface. In the case of transition metal oxides, oxygen atoms typically sputter at a higher rate than the metal atoms, leading to reduction of the metal’s oxidation state. As outlined in Reference 3, higher valent oxides tend to reduce to common suboxides, although a mixture of oxidation states is typically found even after very long sputtering times. Some of the oxide may even be fully reduced to elemental metal. These chemical changes severely limit the use of ion sputtering in the study of oxide films on metal surfaces.
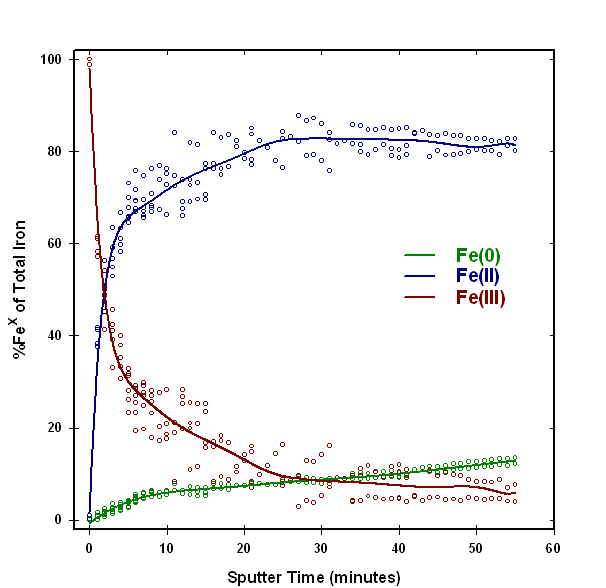
Figure 3 shows a sample of hematite (Fe2O3) powder that was analyzed to show the effects of ion sputtering on iron oxidation states. The red hematite powder was pressed into indium foil, and then analyzed in a Physical Electronics Quantum 2000 XPS system. The dark square is the analysis area, where the sample was analyzed after sequentially sputtering with a 1.0 kV argon ion beam. A plot of iron oxidation states as a function of ion sputtering time if shown in Figure 4. The analysis was repeated several times, and the solid line was fitted to the average of the multiple analyses. Figure 4 shows that most of the iron (III) surface atoms in hematite are rapidly reduced to iron (II), and some atoms are completely reduced to metallic iron. These effects are responsible for the color change in the analysis area seen in Figure 3. The immediate and extensive reduction shown by this example illustrates that ion beam sputtering cannot be used in the analysis of the valence of iron oxide samples.
References
1. Rhodes, K. Application Note: Analysis of Iron Oxidation States by XPS. Modern Microscopy online journal, December 1, 2005. (Date accessed: December 1, 2005).
2. Hoffman, S., in Practical Surface Analysis, Second Edition, Volume 1-Auger and X-ray Photoelectron Spectroscopy, Wiley, Chichester, UK (1990).
3. Barr, T.L., Modern ESCA, CRC Press, Boca Raton, FL., 1994.

Comments
add comment