Micro-Extraction of Soluble Components from Small Particles for Infrared Analysis
Introduction
In a laboratory such as ours, very small particles are received for identification every week. Light microscopy and infrared analysis followed by SEM-EDX or Raman spectroscopy will normally suffice for identification of the major components plus many of the minor ones in a particle. However, organic components present at concentrations of less than 10% can be difficult to identify by infrared and may not be observed. This paper will discuss a simple and quick micro-extraction technique that can isolate and concentrate small volumes of organics which may be present in particles.
Applications of this micro technique include separation and identification of binding material in pigment particles in small paint chips and isolation and identification of minute quantities of lubricant in wear particles.
Ideally, as little as 0.1% of a 0.1 mm diameter particle can be separated using this technique and analyzed by infrared spectroscopy.
The Micropipette
The micro extractions are performed with polyethylene micropipettes that can deliver nanoliter-scale drops of solvent precisely and reliably, using capillary action, when the tip of the micropipette is touched to a smooth surface. The size of the drops varies greatly with the tip diameter and the amount of solvent in the micropipette. Figure 1 shows the dimensions of the micropipette, the various tip sizes, the volume of the drops, and the approximate spread of those drops on KBr or other polished surfaces at room temp. Other micropipette characteristics have also been included in Figure 1.
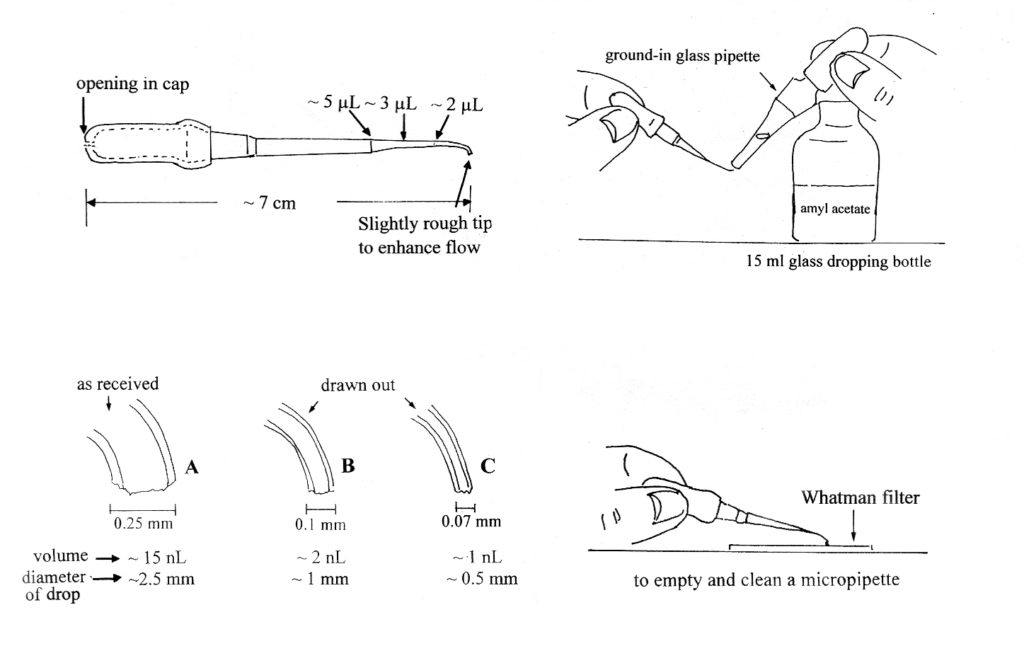
Figure 1.
Some interesting micropipette properties:
- A colorful cap, which helps to locate the small pipette on the bench, must have an opening so the pipette will dispense drops only by capillary action.
- The pipette is filled by capillary action as well.
- Tip B dispenses the correct volume for extracting soluble components.
- The same pipette is used for all solvents (except silicone). It is rinsed a few
times with the new solvent before it is used in the next extraction.
Micropipettes may be made from high density polyethylene tubing or they may be made from low density 1-100 nL Eppendorf ™ Geloader Tips, as described in The Particle Atlas1 and in The Microscope2.
Other Tools and Solvents
The micro solvent extraction is performed under a stereomicroscope equipped with coaxial illumination, a transmitted light base and up to 50X magnification.
In addition to the micropipettes, the following tools and supplies are needed:
-
- Fine to medium tipped tungsten needles1,2
-
- Polished KBr crystals
-
- Low E-glass slides
-
- Aluminum-coated slides or other infrared-appropriate substrate
- A silicon wafer, ideally marked with a 1 mm grid (to be used to check solvent cleanliness)
A set of frequently used organic solvents for extraction such as amyl acetate and nonane should be available. Amyl acetate and nonane both possess medium volatility and low to medium surface tension. Highly volatile solvents may be difficult to work with, as they flash off too quickly. It should be noted that some solvents contain non-volatile impurities which may interfere with micro-analysis and should be pre-filtered, if this is the case.
Micro Extraction of a 0.1 mm Diameter Particle
The extraction technique is illustrated using amyl acetate, which is a very versatile solvent, on a 0.1 mm diameter particle. Amyl acetate has a medium to low surface tension and spreads well on KBr. A 1 nL drop of the solvent takes 2-5 seconds to evaporate, which gives the analyst sufficient time to observe the extraction and note if the drop size should to be changed. A list of solvents with their approximate evaporation rates is listed in the book by H. J. Humecki3. Extractions using a high surface tension solvent, such as water, require slightly different handling and will not be covered in this paper.
The pipette is first filled with amyl acetate to the 2.5 µL mark. The size and the cleanliness of the dispensed drops are checked on the silicon wafer under the stereomicroscope using coaxial illumination. The drops should spread from 1 mm to 2 mm and will evaporate in 2-5 seconds. Drop size can be changed by about 0.5 mm if the volume of solvent in the micropipette is increased or decreased. A drop that leaves a visible residue indicates an unclean solvent, pipette or silicon surface. All three may need to be checked and corrected before proceeding with the extraction.
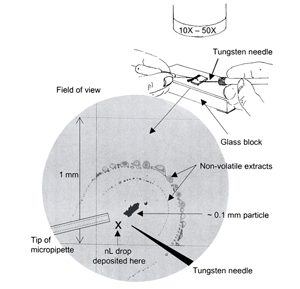
The extraction is performed directly on the substrate being used for infrared analysis, such as KBr or other salt crystal, aluminum coated slide, or low E-glass slide. Using the stereomicroscope with either coaxial or transmitted light, a 1-2 nL drop of amyl acetate is placed directly below and slightly to the side of the 0.1 mm particle being examined. The solvent drop will spread evenly in all directions and the portion that goes through the particle will carry away any amyl acetate-soluble fraction. When the drop reaches its maximum spread and starts to go to dryness, it will leave behind the non-volatile extract in a semicircle around the particle. The solvent may take 2-5 seconds to evaporate. The extraction steps are repeated a few more times to be sure that the soluble fraction has been completely removed from the particle.
The above steps are illustrated and diagrammed in Figure 2. The extraction illustrated in the photograph was performed on a gridded silicon wafer for better photography. The thin film of extracted material shows up well on the smooth reflective surface and the mm grid indicates just how far the 1-2 nL drops have spread.
Concentration of the extract
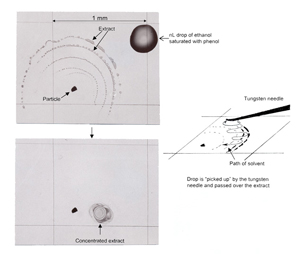
If the extract has spread too thinly to give a good infrared spectrum it can be concentrated with a nL drop of the same solvent held beneath a slightly curved tungsten needle as shown in Figure 3. Note that a solvent drop held beneath a needle will take longer to evaporate than a drop allowed to spread freely on a surface. Again, the soluble components will be dissolved in the amyl acetate. As the amyl acetate evaporates, the soluble fraction will remain beneath the needle. Some of the soluble fraction will adhere to the needle but most will remain on the substrate, concentrated in an area 50-200 µm across.
Variations of the extraction technique
Frequently, the extraction is performed with two sequential solvents. For example, a heavily filled adhesive contaminated with a lubricant can be treated as follows. Nonane is used first to extract the lubricant. The particle is then moved over a few millimeters and the adhesive is extracted using amyl acetate. The inorganic filler can be submitted for EDX analysis or pressed into a thin film and analyzed by infrared as well.
Concentration of the extract can be performed with a solution of ethanol saturated with phenol crystals. It is an excellent solvent for concentrating extracts on KBr surfaces because it has a high surface tension and the drops will not spread but will remain as small as 0.2 mm across, and will take longer to evaporate. With the longer evaporation time, the analyst has time to concentrate the extract into an area less than 0.2 mm.
In Summary
By mastering the micro-extraction technique, the analyst can, in only a few minutes, extract pure components from a non-homogeneous 0.1 mm particle and make infrared identification easier and more accurate.
References
1. McCrone, W. C. and J. G. Delly, The Particle Atlas, Ed.2, Vol. 1 (1973), pp 228-229.
2. The Microscope, Vol. 47 (1999), pp. 63-67.
3. Practical Guide to Infrared Microspectroscopy, H. J. Humecki, Ed., Marcel Dekker, New York, 1995.
Acknowledgement
The author gratefully acknowledges Dr. Kathleen Martin, Senior Research Chemist, McCrone Associates, Inc. for her assistance in producing this paper.
Comments
add comment