21 CFR Part 11 Compliance for your Pharmaceutical Microscopy Lab
Many years ago the Food and Drug Administration (FDA) enacted regulation 21 CFR Part 11, regarding electronic records and electronic signatures. This set of rules states the requirements for electronic signatures and records, and outlines the management of records in electronic quality management systems for life science, pharmaceutical science, and other FDA-regulated industries. All laboratory computer systems which store quality data must be compliant with 21 CFR Part 11. In addition, any system that is used for reporting data to the FDA must also be 21 CFR Part 11 compliant, for example, a pharmaceutical company that stores microscopy images, measurements, or quality data from laboratory results.
Meeting 21 CFR Part 11 requirements is made simple with PAX-it Extended Security software. For over 25 years, PAX-it software has provided image analysis and management tools which allow the user to measure and annotate images, create reports, and store digital assets. Additional layers of protection within the Extended Security module ensures compliance with FDA regulations. These additional layers include:
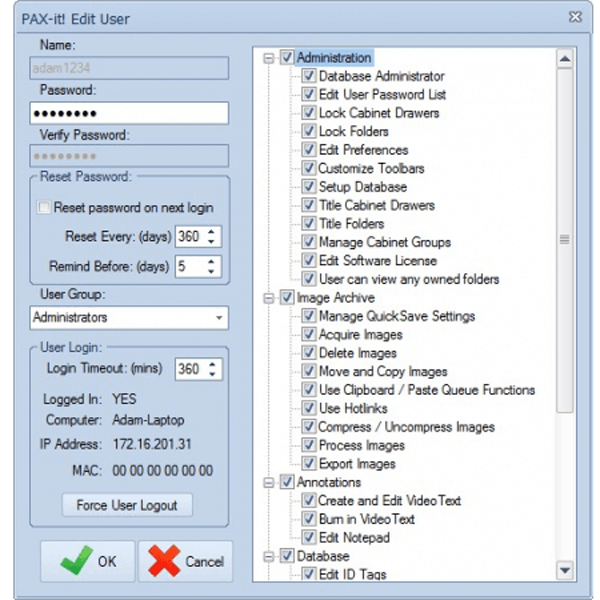
- Administrative Tools: Assign unique logins to users accessing the PAX-it application software. Logins may be created within PAX-it itself or synched to Windows Active Directory for a more streamlined approach to user management and authentication. An extended list of PAX-it permissions enables administrators to regulate the actions each user can take—from the assignment of user-owned folders and view-only permissions, to limiting access to specific functions, such as annotating or calibrating.
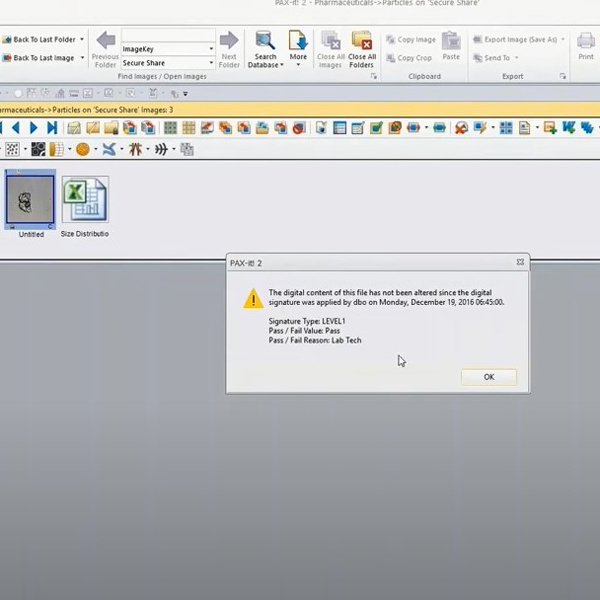
- Electronic Records and Electronic Signatures: Easy database storage and digital file management is the true advantage of PAX-it software. The Extended Security module brings this same ease of use to digital signatures—a core requirement for 21 CFR Part 11 compliance. Digital signatures can be applied, locking the image, report, or data so that it may not be modified. Authentication tools are available to verify the integrity of the asset, with built-in warnings to alert you if any original or signed file has been altered after insertion into PAX-it software.
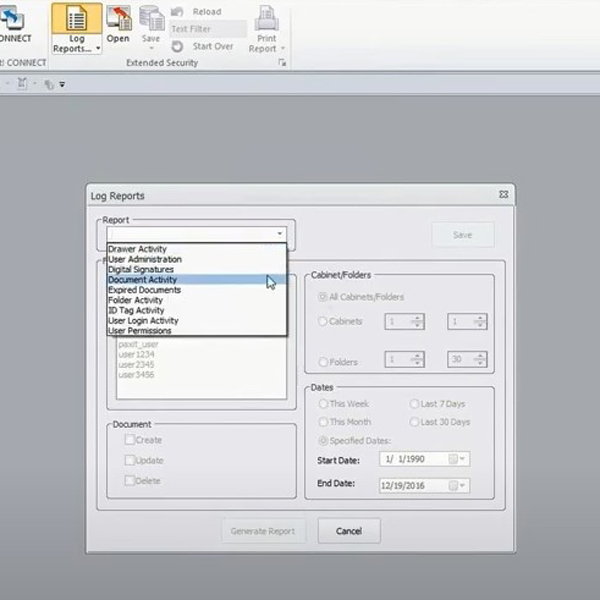
- Detailed Audit Trails: Administrators are allowed to track activity related to image manipulation, including moving, deleting, and adjusting. The creation and modification of other file types, including reports and presentations, are logged. Audit trails are sortable by the image activity, by user activity, by date, and more.
McCrone Microscopes & Accessories is an official PAX-it software dealer and we can provide a complete solution, including software and IQ/OQ/PQ services. Let us help you meet 21 CFR Part 11 compliance.
Comments
add comment