Contaminant Identification in Pharmaceutical Products
This article is republished from The Microscope, Vol 51:1, 3-10 (2003)
Abstract
Most pharmaceutical products are specified to be essentially free of visible particles and there are limits on the number of subvisible particles allowed. The FDA requires that contamination problems are fully investigated in a timely fashion. This paper describes an analytical approach that utilizes microscopical examination coupled with sample isolation, preparation, and analytical methods optimized for small particles, to successfully identify particulate contamination for regulatory compliance.
The analytical approach for the identification of particles will be outlined. The pharmaceutical product analysis plan includes sample examination, gathering of background information concerning the sample and particle isolation. Analytical methods utilized include one or more of the following: polarized light microscopy (PLM), Fourier transform infrared spectroscopy (FTIR), Raman Microscopy, and several types of electron microscopy with EDS and WDS detectors. Common contaminants in pharmaceuticals include natural and synthetic fibers, silicone, plastics, rubber, metal particles and corrosion products, glass particles and delamination flakes, skin flakes, char particles, detergents, lubricant oils, Teflon® and graphite. Specific examples of product-related particulates, foreign particles and manufacturing process contamination will be discussed.
Do you need assistance with a materials or particle contamination issue? Speak with a scientist.
Introduction
Identification of small particle contamination is crucial in the pharmaceutical industry. Most pharmaceuticals are specified to be essentially free of visible foreign particles and there are limits on the number of subvisible particles allowed. Contaminants are usually first noticed during internal quality control inspection; however, some are detected by the consumer after the material has been released. The FDA requires that contamination problems and consumer complaints are fully investigated in a timely fashion. Microscopical analysis is particularly well suited to the analysis of particulate contamination because the particles are usually too small to be analyzed using conventional methods. Proper methods of sample isolation and preparation are also critical to the successful pharmacuetical contamination identification.
The most common pharmaceutical samples that are analyzed by McCrone Associates are filled and unfilled vials (parenteral products) and tablets; however, particles and residues have been isolated from syringes, IV bags and tubing, ampoules, dropper bottles, inhalors and patches. Foreign particles also may be recovered from raw materials and various types of process filter apparatus.
There are two general types of material. The contaminant may be related to the active ingredient, excipient materials or colorant. Particles may be generated from the product container or packaging material. These types of particles include glass, rubber, aluminum, plastics and paper. Contamination can also result from the manufacture of the product; examples of these include charred product, detergents and lubricant oils. Metal and metal corrosion, Teflon, graphite and rubber particles are indications of tank, filter or equipment failure. Environmental contaminants such as fibers and skin cells are also found. The most common contaminants in pharmaceuticals are cellulose (cotton and paper) fibers, synthetic fibers, silicone, plastics, rubber, metal particles and corrosion products, glass particles and vial delamination flakes, skin flakes and char particles.
Analytical Method for Identification of Small Particles
The first step in the analysis is to obtain as much background information about the particulate problem as possible. This is important for both in-house investigations as well as for samples submitted to an outside laboratory. In some cases, clients are not forthcoming about the details of the problem, because they do not wish to bias the investigator. This can cause longer turn around time and increased costs. Communication with the client is critical to defining the analysis. It is also useful to know the compositional information of the product, as this knowledge may aid in the interpretation of the results. Some information can be obtained from reference volumes, such as the Physicians Desk Reference (PDR) and The Merck Index. It is impossible to underestimate the importance of gathering all available background information about the sample.
Particle identification begins with microscopical examination. The sample is examined as received, usually using a stereomicroscope. The optical examination further defines the analysis, and a plan of action can then be developed. The next step is to isolate the contaminant and prepare it for further microscopical and chemical analysis. Obviously, it is very important to avoid introducing any further foreign particles into the sample during isolation. To avoid this, the sample should be isolated in a cleanroom facility, if possible, or in a laminar flow hood. McCrone Associates has a 1650 sq. ft cleanroom facility. The facility is equipped with microscopes, analog and digital camera equipment, Class 100 laminar flow hoods, and a deionized, particle-free water source. Particles in solution are isolated by filtration. Polycarbonate membrane filters are normally used because they have a smooth surface from which to observe and pick particles. Contaminants on or in tablets can usually be recovered by picking directly with a tungsten needle and moving it to a suitable substrate for analysis. Microextraction techniques are useful for isolating oils from defects. Information on the manipulation and preparation of small particles can be found in several references (1, 2, 3, 4).
After the particles are isolated, they are prepared for further analysis using one or more methods. The appropriate method or methods are chosen based on the optical examination of the sample. Sometimes one method is sufficient, other particles require additional analysis to fully characterize the contamination. Frequently particles are initially examined using polarized-light microscopy (PLM). Some particles, such as fibers, may be identified readily using light microscopy; other particles are prepared for additional techniques based on characteristics noted by PLM.
Fourier transform infrared spectroscopy (FTIR) is a technique that is widely used to identify organic and some inorganic materials. FTIR microscopy is recommended for particles that appear to be organic during the optical examination. Organic particles include polymeric material, amorphous residues and particles that appear to be related to active ingredients and excipients. Some inorganic materials and minerals, such as calcium carbonate or clay, can also be characterized and/or confirmed by FTIR. Raman microspectrometry is a complimentary method to infrared, but it is an emission technique that is particularly well-suited to the analysis of dark and opaque particles, corrosion products and minerals. It can determine the phases of carbon (char, graphite) and is particularly useful for pigments.
For elemental analysis, a low vacuum scanning electron microscope that is equipped with an energy dispersive x-ray spectrometer (EDS) detector is utilized. The low vacuum capability is very useful for contaminants that consist of an organic matrix with inorganic inclusions. The low vacuum mode can be used to minimize charging effects. SEM imaging also provides high magnification morphology data, and backscattered electron imaging allows elemental mapping. For identification of specific elements that may be masked or overlapped by another element, an electron microprobe that has both EDS and wavelength dispersive x-ray detectors (WDS) may be used. Analytical electron microscopy or transmission electron microscopy may be used for submicrometer particle analysis.
For certain applications, other methods such as secondary ion mass spectrometry (SIMS) may be used. SIMS is very useful in detecting light elements that cannot be detected by EDS. Gas chromatography coupled with a mass spectrometer (GC-MS) can be used if the contaminant is soluble in an appropriate solvent and is volatile. Although not necessarily considered microanalytical techniques, time of flight secondary ion mass spectrometry (TOF-SIMS), direct probe pyrolysis mass spectrometry, and liquid chromatography-mass spectrometry (LC-MS) have been successfully used in situations where there was sufficient sample available.
Examples of Pharmaceutical Contamination
Fibers
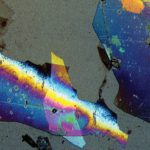
Fibers are a commonly encountered contaminant. Fibers are usually identified using polarized light microscopy alone, but FTIR is sometimes used to confirm the chemical identification. Sometimes, SEM is used to examine the fiber morphology. Most often, paper, cotton, and colorless polyester fibers are observed in pharmaceuticals. These fibers are related to cleanroom wipes and garments. Sometimes, a variety of fibers is found within the same sample, and this is an indication of a more serious contamination issue. Figure 1 shows some typical fibers recovered from a pharmaceutical product.
Glass
Glass is another contaminant that is often observed during quality control screening. Glass particles can be generated by fracture of the vial neck or opening, from external sources such as other vials, glassware and lighting, and from delamination of the inner vial surface. Because of their density, glass particles sink rapidly to the bottom of the vial when the liquid is agitated. Glass delamination flakes are extremely thin and may be missed during visual examination. If a larger number are present, a “twinkling” effect in the solution is observed. The “twinkling” is best observed using a fiber optic light source. Delamination flakes do not sink to the bottom of the vial. Glass delamination usually occurs when a highly acidic, highly basic or sodium chloride solution is stored in the wrong type of container.
Glass particles have distinctive morphology and may be identified by microscopical analysis. Figure 2 shows several glass particles on a polycarbonate membrane filter. The glass particles are shiny, refractive and have a characteristic conchoidal fracture. When examined using polarized light, glass is isotropic. Refractive index measurement and elemental analysis can be used to determine the type of glass and to compare it to suspect sources. The elements lithium and boron are present in some types of glass, and these light elements can not be detected using EDS detectors; if detection of these elements is needed, the particles can be analyzed using secondary ion mass spectrometry (SIMS).
Glass delamination flakes as they appear on the filter membrane are shown in Figure 3. Using correct illumination is critical. Due to their extreme thinness, the flakes would not be observed on a membrane filter using oblique or side light; they are only seen using episcopic illumination or top light. If glass delamination flakes are detected, the interior of the sample container should also be examined microscopically. The beginnings of delamination are observed as small pitted areas resembling circles and doughnuts on the interior glass surface. A vial interior exhibiting a severe case of delamination is presented in Figure 4. The heaviest delamination tends to occur at the bottom sides of the vial, and lessens further up the vial sides. In Figure 5, the fill line of the vial is evident.
Silicone
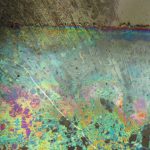
Silicone is used in many products and it is frequently found in pharmaceutical products. When it is used as a lubricant for rubber stoppers and plungers, it very easily “sloughs off” and ends up in the product. Silicone can interact with protein based drugs or active ingredients producing particles or residue. Thermal degradation of the silicone can occur if the product is autoclaved. Silicone oil can be observed in liquid products as oil droplets. Sometimes it occurs as fine droplets that give the solution a hazy appearance. After filtration of the product, silicone oil appears as “cleared” areas on the filter that can be observed using transmitted light. Figure 6 shows the cleared zones on a polycarbonate filter. Degraded silicone, or silicone that has interacted with protein or active ingredient, forms a semi-solid particulate residue that appears as stringy “fiber-like” particles in solution. Stringy silicone particulate, as it appears on the membrane, is shown in Figure 7. It is essential to use the correct type of illumination (epi-illumination in this case), as this type of residue often cannot be seen with transmitted or oblique illumination.
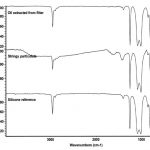
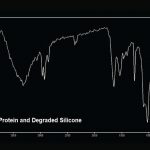
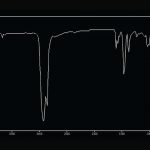
Suspect silicone residues are prepared for analysis using FTIR to confirm the identification. The silicone oil can be recovered from the filters with oily or cleared zones by extracting a small portion of the filter with nonane. This procedure is performed onto a polished salt plate for FTIR analysis. Stringy residues can be scraped off the filter using a fine tungsten needle and prepared for FTIR. Small amounts of silicone residue can be prepared for analysis using special replication techniques. Infrared spectra of silicone oil and stringy silicone particulate from pharmaceutical products are compared in Figure 8, and a typical spectrum of silicone/protein residue is presented in Figure 9.
Vials
Particles and residues can occur as a result of processing and sometimes are observed on the vials prior to filling. Figure 10 shows a residue from an unfilled vial. FTIR analysis indicated that the material was similar to the processing detergent (Figure 11).
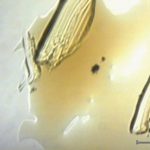
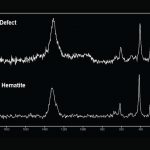
Occasionally, a vial will be submitted that was thought to contain a dark particle. Optical examination indicates that the defect or particle is, in fact, embedded in the glass or is contained entirely within the wall of the vial. Figure 12 shows black material that is embedded in the vial glass. The material was identified as hematite (iron oxide or rust) using Raman microscopy (Figure 13). The presence of iron and oxygen can be confirmed using EDS. Raman has the benefit of giving compound information that complements the elemental data.
Tablets
Contamination on tablets is well addressed by microscopical techniques. Some surface contaminants are actually a clump of ingredient that is not properly dispersed. Gray or black spots may be caused by a number of tiny metal particles (wear or corrosion particles) that are mixed with tablet material causing a dark particle. Tan or brown particles on or in tablets are frequently found to be thermally degraded (charred) excipient material.
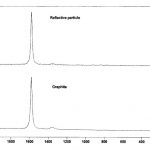
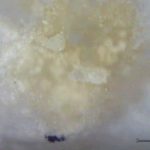
Using Raman microscopy, surface defects may be analyzed directly with no sample preparation needed. One example involved a tablet that supported a number of silver reflective particles on the surface that initially appeared to be metallic. EDS analysis indicated only carbon and oxygen were present. Light microscopical analysis at high magnification showed that the reflective particles had a platy morphology that was consistent with graphite. In situ Raman analysis confirmed the presence of graphite (Figure 14). Graphite can be easily distinguished from other phases of carbon by the strong, sharp band that occurs around 1590 cm-1. Defects that are caused by processing agents, such as lubricants on tablets, are sometimes difficult to identify. The lubricant is intermixed with the tablet material and an infrared spectrum of the defect material contains bands due to the tablet excipients in addition to the contaminant. The bands due to the contaminant frequently are masked by those of the tablet material. Lubricant oils may be isolated from tablets using microextraction techniques. The tan discoloration on the surface of a tablet (Figure 15a) was extracted using a glass capillary micropipette and solvent. The FTIR spectrum of the extracted material is shown in Figure 15b. The primary bands are characteristic of hydrocarbon oils.
Yellow stains on tablets are somewhat common, but are usually problematic to identify. The discoloration can vary from a faint tan to orange yellow. The cause of the discoloration is frequently difficult to detect analytically. In a few cases, evidence of an active ingredient other than that found in the tablet has been detected. One particular tablet supported a yellow stain over almost the entire white coating. Initial FTIR analysis of the yellow stain indicated only components that could be attributable to the coating. A second stereoscopic examination of the tablet indicated that there were some orange spots scattered on the surface. The FTIR spectrum of an orange spot within the yellow coating was consistent with that of sulfasalazine. There is a published report (5) of orange stains on Daypro® tablets that were identified as sulfasalazine. This active ingredient was not present in the manufacturing facility where the tablet was produced. It was deduced that the tablets were contaminated with sulfasalazine dust in a dispensing tray at a pharmacy. The article (5) may be consulted for further information concerning the Daypro investigation.
Summary
Successful microscopical analysis of particulate contamination begins with good communication between analyst and client. The optical examination, using a stereomicroscope and a polarizing light microscope, enables the analyst to characterize the contaminant and chose the appropriate sample isolation technique. Analytical methods that are optimized for small particle analysis, such as FTIR microspectrometry, Raman microspectrometry and SEM/EDS permit chemical identification of particles that are too small for conventional chemical analysis. The results of the optical examination, coupled with the chemical data, usually enable the client to identify the source of the pharmaceutical contamination.
References
Teetsov, Anna S. (1995) Unique Preparation Techniques for Nanogram Samples, in Practical Guide to Infrared Microspectroscopy (H. Humecki, ed.), Marcel Dekker, Inc., New York, 417-443.
Teetsov, Anna S. Preparation and Use of Needles and Micropipets for Handling Very Small Particles, The Microscope, 1999, 47, 63-70.
Teetsov, Anna S. An Organized Approach to Isolating and Mounting Small Particles for Polarized Light Microscopy; The Microscope, 2002, 50, 159-168.
McCrone, W. C. and Delly, J. G. The Particle Atlas, Ed. 2, Vol. 1, 1973, 228-229.
Schmann, Steven C. Investigation of Product Complaints About a Prescription Drug: Implications for Pharmacy Practice and Development Pharmaceutics; Journal of the American Pharmaceutical Association, 2000, 40, 89-92.
Acknowledgements
The author gratefully acknowledges Joe Barabe, John Delly and Bonnie Betty for their assistance in preparing this paper. The author also thanks Dr. Kenneth Smith and Barbara Blaum for their contributions to the manuscript.







Comments
add comment