A Microscopical Investigation of Pigments and Technique in the Cézanne Painting “Chestnut Trees”
Editors’ Note: In this second article by Marigene H. Butler, the methods and techniques described in her first, basic article are applied to an actual painting. The original article was presented at the First Annual Meeting, American Institute for the Conservation of Historic and Artistic Works, Inc., May 31-June 2, 1973, at the William Rockhill Nelson Gallery of Art, Kansas City, MO. This article, and others at the time, consisted of a compilation of mimeographed paper stapled together, which did not have wide distribution. We are, therefore, pleased to make this invaluable information available to a wider audience, as a template for the identification of paint pigments, and the investigation of painting technique.
At the time of the writing of this article, Marigene H. Butler was the Director of the Intermuseum Laboratory, Oberlin, Ohio, providing conservation services to the seventeen Midwestern museums of the Intermuseum Conservation Association. In 1978, she became Head of Conservation at the Philadelphia Museum of Art, where she was responsible for planning and implementing conservation care and treatment for the entire museum collection, with a special expertise in the treatment of paintings; she retired from there in 1997.
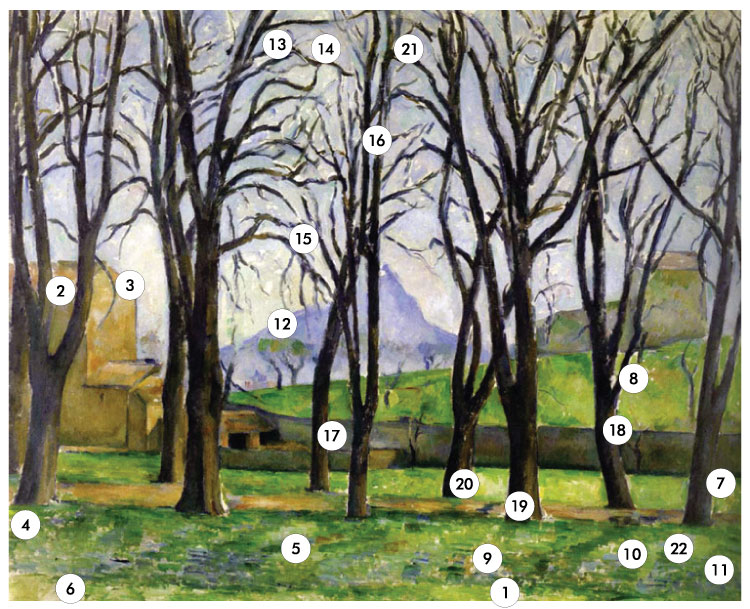
The Cézanne painting Chestnut Trees (1) was examined in order to learn as much as possible about how it had been created. The information sought included what pigments had been used, how they had been combined to produce the various design tones, and how the paint had been applied, both to model form and to create the recession of planes in space.
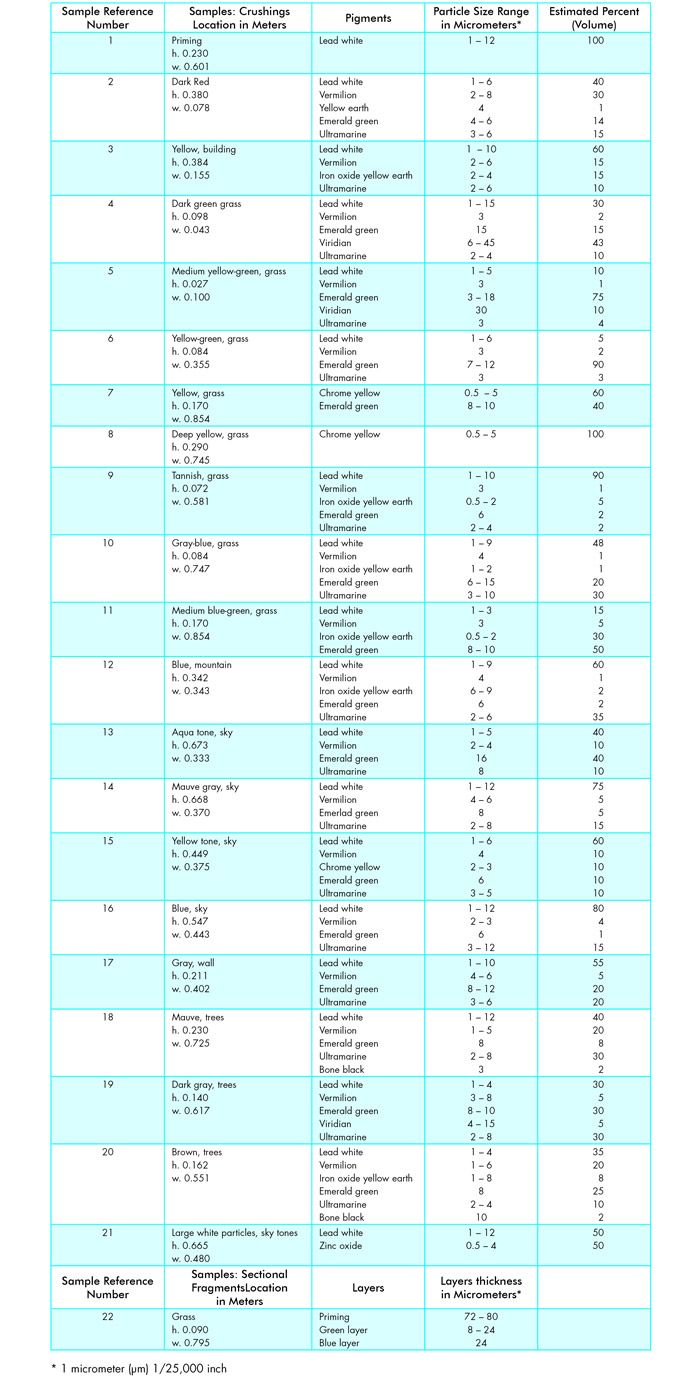
This investigation began by using a low power stereomicroscope to study the brush work, handling of paint, and variety of tones used in the design area. Samples were then taken from 20 different tones of paint (2), the paint particles were dispersed, mounted in Aroclor® 5442 (3) (refractive index 1.66) and identified using a polarizing microscope. Identifications were made by using characteristic optical and physical properties. The optical properties included relative refractive index, isotropy and anisotropy, birefringence, and type of extinction. The physical properties included color in plane polarized light and between crossed polars, degree of transparency or opacity, size, shape, surface texture, cleavage, fracture, homogeneity, and degree of agglomeration. Pigment particle size ranges were measured and the percentages of pigments in a mixture were estimated by volume. Apochromatic objectives were found to be essential for studying these samples, especially for seeing the identifying properties of pigment particles because they most frequently fall into a very low 0.5-10 µm range. One sectional fragment was taken from the painting and mounted in viscous Aroclor 1260 for the purpose of measuring typical paint layer thicknesses.
Pigments Used in the Painting and Their Identifying Optical and Physical Properties
Eight pigments were found to have been used in this painting: lead white, vermilion, chrome yellow, iron oxide yellow earth, emerald green, viridian, ultramarine, and bone black.
Lead white was used as the priming material and appeared in nearly all of the paint tones. In both priming and paint layers it occurs in 1-12 µm particles, much larger than are usually found in paintings. When identifying lead white, it is useful to have in mind the fact that crystals of basic lead carbonate are in the hexagonal system and are therefore uniaxial. Thus they have two refractive indices and are anisotropic. Because of their relatively high birefringence, (–)0.15, related to their particle sizes, smaller particles glow with bright white to straw yellow first-order interference colors, and larger particles show various second-order colors when viewed with polarized light. The frequent agglomerates have a pearly-pink cast. In plane polarized light, particles are transparent to translucent, colorless to white, with tannish-gray tones. The irregularly rounded particles can sometimes be seen at 1000X to be thin scales or tabular with hexagonal shapes. As a component of pigment mixtures in this painting, lead white ranges from 5 percent or 10 percent in the yellow-green grass tones to 40 percent of the dark red tone, 60 percent of the yellow building tone, and 40 to 80 percent of various sky tones. Large white agglomerates are visible at 16X, especially in the sky tones. When some of these were crushed, they turned out to be lead white with areas of zinc oxide particles, identifiable by their very low birefringence, (+)0.02, and refractive indices above 1.66. This was confirmed by electron microprobe x-ray analysis. It is possible that the agglomerates of lead white and zinc oxide were formed during the manufacturing process.
Vermilion is also present in nearly every pigment mixture, but usually only a few particles, one percent to five percent. Exceptions are the dark red tone with 30 percent and the mauve tree tone with 20 percent. These red crystals of vermilion, which also are in the hexagonal system, are likely to be rhombohedral, thick tabular, or prismatic with perfect prismatic cleavage and uneven subconchoidal fracture. With refractive indices well above 1.66 and a high birefringence of (+)0.33, these crystals are most readily recognized by the yellow-to-orange colors of smaller particles and the orange-red of larger particles in polarized light. Vermilion differs from the other inorganic red pigments in its polarization colors and in the rounded prismatic shapes of its particles which have very irregular surfaces and appear to glow with a bright, orange-red light which tends to mask the conformation of the surfaces. As a further test for identification, particles of mercuric sulfide will round off when heated on a slide over an alcohol lamp. If heated to 583°C, they sublime and droplets of mercury will collect on the coverglass. However, with practice and comparison with particles of the same size in reference standards, this confirmation is rarely needed for identification of particles in a dispersed crushing.
Chrome yellow occurred in four samples, ranging in quantity from 10 percent in the yellow sky and tannish grass tones, to 60 percent in the yellow grass, and 100 percent in the deep yellow grass tones. These translucent monoclinic crystals of lead chromate, with refractive indices above 1.66, occur as minute 0.5-1 µm spheres and 2-5 µm rods and are most readily identified with crossed polars when they show grayed-yellow to bright-yellow colors.
Particles are pleochroic with alpha and beta orange-red and gamma blood red, but because they are so small, at 1000X in plane polarized light their pleochroism can barely be seen to change from bright yellow to orange as the stage is rotated. Particles often form loose agglomerates which are an orange-yellow. In the tones containing only 10 percent of chrome yellow, it was necessary to use the 100X/1.30 oil planapochromatic objective in order to distinguish these particles from the remainder of the crushing. With crossed polars, the rod-shaped particles then were visible.
Iron oxide yellow earth occurs in seven samples, ranging from a few particles, one percent in the dark-red or blue mountain tones, 15 percent in the yellow building, and 30 percent in the medium blue-green grass tone. The yellow earths consist of the mineral goethite whose crystals belong to the orthorhombic system and have refractive indices greater than 1.66. Two types of particles are present in these samples. The larger ones, up to 9 µm, appear to be isotropic except for occasional anomalous birefringence due to impurities. In plane polarized light, these occur as irregularly rounded nodules or fused agglomerates of a translucent, brownish-yellow color. Also present in several samples are 0.5 µm oval particles which are most readily seen between crossed polars when they flicker with pale to bright first-order colors.
Emerald green, a pigment which is especially intriguing visually, turns up in nearly every tone, ranging from a few particles in the yellow sky and dark red tones to 30 percent in the mauve sky tone, 50 percent in the blue-green grass tone and 75 percent in the medium-yellow grass tone. This pigment is copper acetoarsenite with refractive indices above 1.66. Uncrushed particles, 8-15 µm in diameter, appear to be radial in structure and usually have a dark spot at the center, causing them to resemble a doughnut. When these are crushed, they separate into several dozen rounded or wedge-shaped flakes. In plane polarized light, the thin flakes are transparent and nearly colorless, while the translucent, intact particles show pleochroism, changing from pale yellow-green to a grayed blue-green as the stage is rotated. With crossed polars, the thin flakes show first-order gray to white colors and intact spheres glow brightly with a creamy yellow to yellow-green to gray-blue. Undulose extinction is evident as the stage is rotated and flickering dark areas move irregularly across particle surfaces. Also present in these samples and in reference standards of emerald green are 9-12 µm fused agglomerates of 0.5 µm transparent birefringent particles with refractive indices above 1.66. The precise composition of these particles has not yet been determined. When thin emerald green particles are mixed with other pigments in a sample they can be hard to distinguish. Using slightly uncrossed polars to see the green color or fully crossed polars to see the undulose extinction is usually helpful.
Another green pigment, viridian, or transparent chromium oxide, with refractive indices above 1.66, occurs as five percent of the dark gray of the trees, 10 percent of the medium yellow-green grass, and 40 percent of the dark green grass. In plane polarized light, these transparent to translucent particles are pale to deep watery aqua-green, depending upon their thickpess. With crossed polars, thin particles show first-order gray to white colors and thicker areas are green. As particles are rotated, undulose extinction causes light and shadow areas to flicker irregularly across their surfaces. Particles in this painting are large, 6-45 µm, and tabular, often present in jagged blade-like fragments with irregular shapes and fractured edges.
Ultramarine blue is the only blue used in this painting. Its translucent particles are in the cubic system and therefore are isotropic. Its surest identifying characteristics are its isotropism, its deep purple-blue, and its refractive index which is lower than 1.66. These 3-10 µm particles are very likely artificial ultramarine, judging from their irregularly rounded shapes, rough surfaces, fairly uniform sizes, and dark color. In samples from this painting they occur singly, never in agglomerates. Ultramarine particles are present in nearly every tone sampled, ranging from two to 15 percent in the dark red, yellow, and green tones, to 30 percent in the gray-blue grass, and 35 percent in the blue mountain tones.
Finally, a very few particles of bone black are present in the brown and mauve tree tones. These opaque particles of charred bone have irregular shapes and jagged edges and range from 3-10 µm. It is significant to note how little black pigment Cézanne used in this painting.
Cézanne’s Technique in Chestnut Trees
Table I, which lists the pigments, proportions, and particle sizes of the twenty samples, shows an interesting fact. Sixteen of these samples contain lead white, vermilion, emerald green, and ultramarine, but the proportions of each of these pigments are varied, creating differences from tone to tone. For example, the dark red tone includes these four pigments with a high 30 percent of vermilion. The aqua sky tone is 40 percent emerald green. The mountain blue tone is 35 percent ultramarine, and the mauve tree tone is high in both ultramarine and vermilion. These subtle variations of the same pigment combination perhaps help to explain the harmony between all of the tones used in the painting.
One sectional fragment was taken (see Table I) and it was difficult to find an area for this purpose where two layers of paint overlapped each other. Usually one tone was placed next to another tone without any overlapping areas. For example, the wall was painted up to the edge of the tree trunks and the tones did not overlap one another. This placing of different tones next to each other without mixing is used particularly in the foreground and the resulting hard edges give the tree trunks, for example, a solidity that serves to bring them forward in space. Similarly, grass tones in the foreground are painted with separate, short strokes and no blending of adjacent tones. In contrast, the mountain and the trees against the sky have their edges softened by brushing a wet tone into the adjacent wet tone. This gives the illusion of distance and causes the mountain, for example, to fall back in space.
Wet on wet suggests a rapid technique and other evidence for the fact that Cézanne must have painted rapidly, wet paint into wet paint, includes the fact that at low magnification the beginnings or ends of brush strokes such as the tree branches, blend into sky tones beneath them. Sometimes the tree tones are painted over the sky; other times the sky is painted over the edges of branches. The building at the right is another example of this with some blending of adjacent tones which softens the color area, again causing it to recede into the distance. The sky contains at least four tones, blue, aqua, yellow, and mauve, but their variety is minimized by brushing the edges of adjoining tones, wet into wet, into each other; thus the sky tones hold their place in the distance.
Conclusion
The investigation of the materials and technique of Chestnut Trees revealed that Cézanne used eight pigments—a fairly large number. The eight pigments were readily identified with the polarizing microscope by studying their optical and physical characteristics and comparing them with reference standards when needed. By using four of these pigments, lead white, vermilion, emerald green and ultramarine, in varying quantities in nearly every tone, he achieved a harmony of subtle tonal relationships throughout the painting. By placing these varying tones adjacent to one another, sometimes mixing wet paint into wet paint at their edges and sometimes not, he defined the landscape with a recession of planes in space.
A great deal has been learned from this investigation about how Cézanne created one of his paintings. Hopefully this information will be augmented by applying these techniques to the examination and identification of materials in other paintings by Cézanne. (Note: In 1984, the materials and techniques used in 10 paintings from the last three decades of Paul Cézanne’s career were examined by the author; see reference #4.) Such a fund of information provides an understanding of the materials involved which both enhances the conservation treatment of his paintings and aids in distinguishing his work from possible imitations.
References
- Paul Cézanne, Chestnut Trees, 0.733 x 0.925 meters (28-7/8 x 36-3/8 inches). The Minneapolis Institute of Arts.
- Butler, M. H. Polarized Light Microscopy in the Conservation of Painting, Bulletin of the International Institute Conservators, American Group, May 1971.
- Aroclor is a registered trademark of the Monsanto Chemical Company.
- Butler, M. H. An Investigation of the Materials and Techniques Used by Paul Cezanne, American Institute for Conservation: Preprints, 12th Annual Meeting. 20-33 (1984).
Read the next article by Marigene H. Butler.

Comments
Kirsten Moffitt
This is a fantastic article! Thank you for sharing. I teach pigment identification with PLM to art conservation graduate students and I plan on adding this to the class bibliography.
Leslie Bolin
Kirsten, we're glad you found it helpful. I hope you read Marigene H. Butler's other two articles, also. Thank you for letting us know!
add comment