Laboratory Analysis of Stolen Currency
Editor’s note: Craig Schwandt, senior research scientist and director of industrial services, McCrone Associates, performed analysis on two bank notes to determine whether evidence could be obtained that would confirm their presence in the explosion of a bank safe during the 1866 robbery of the Osage National Bank of Osage, Iowa. This article was originally published in the March/April 2016 issue of Paper Money, the official magazine for the Society of Paper Money Collectors, and has been reprinted here with permission from the publisher. Details surrounding the 1866 robbery are given in an accompanying article in that issue.
Improvements in scientific technology and technique have greatly expanded our ability to analyze microscopic samples for their composition, source, and/or age. This has led to various applications, such as the study of chemical impurities, historical artifacts, and crime scene evidence. Although the surface and interior composition of coins has been studied to help determine their history and legitimacy, little similar work has been done on currency. We report here on our laboratory analysis of stolen currency to confirm its well-documented history and to demonstrate the potential for future studies on other currency.
An accompanying article in this issue details the 1866 robbery of the Osage National Bank of Osage, IA, charter #1618. In brief, the door of the bank safe was blown off with gunpowder and among the stolen currency were crisp, unsigned, unissued $5 Original Series notes from the bank with serial numbers 1751 through 2200. The thieves forged the bank officers’ signatures and distressed the notes before starting to pass them. Sixteen of the stolen notes have survived, mostly in tattered and torn condition. On one of the notes pictured below, the stamped letter “S” is faintly visible in blue ink in several places to indicate its having been stolen.
Laboratory Procedure
We studied two of the Osage stolen notes, serial numbers 1951 A and 1966 B. Both notes had been purchased from prominent numismatic sources. Our goal was to determine whether we could obtain evidence confirming their presence in the explosion of the bank safe.

We looked for three different microscopic materials embedded on the surface of the notes. First, gunpowder residue may have survived the explosion. Next, a local newspaper reported that a roll of stolen currency recovered from the thieves was covered with “Plaster Paris.” This white powder was undoubtedly a proprietary fire-proofing material with which manufacturers of bank safes typically lined the safe walls, such as talc, asbestos, or gypsum. Finally, iron fragments would be strong evidence of an explosion.
All laboratory work was done in the facilities of McCrone Associates. The notes were taken into an ISO Class 5 cleanroom. A preliminary examination with an optical microscope revealed very large numbers of particles adhering to the notes. A non-destructive, mildly adhesive film was used to lift particles from the top border of each note. The particles from the lifts were transferred to polished carbon planchets with a very thin layer of dispersed adhesive.

The planchets were inserted into a JEOL variable pressure scanning electron microscope equipped with an Oxford Instruments AZtec energy-dispersive X-ray microanalysis system to examine the composition of a small percentage of particles for each of the notes. This system produced highly magnified images of the particles as well as X-ray spectra emitted by designated particles. The X-ray spectra allowed us to determine the atomic elements present and their respective proportions.
Results
In general, the largest number of particles were carbonaceous compounds, which is not surprising as the bills were heavily soiled. Fibers and skin flakes were also present. Another frequent particle type was various silicate minerals, such as clay, which are common in normal environmental samples.
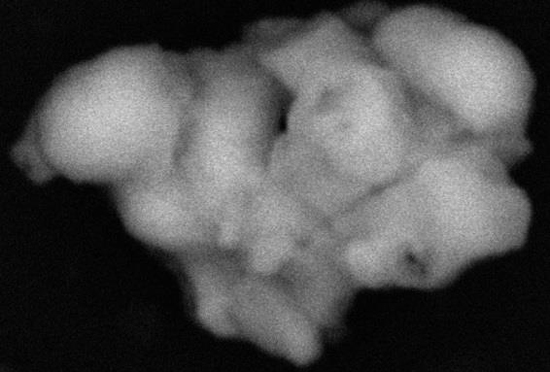
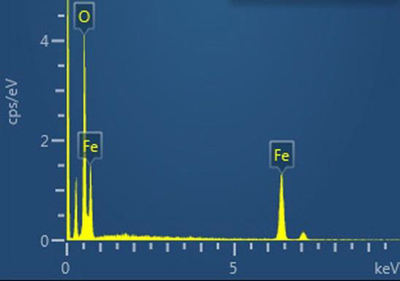
In addition to the common materials listed above, we found some unusual ones. Leading the list were conglomerations of rounded or spheroidal particles of iron oxide, many with traces of silicon and magnesium possibly consistent with a cast iron origin. Some of the iron oxide particles included other trace elements such
as aluminum, phosphorus, sulfur, potassium, calcium, and lead. They were five to ten microns (thousandths of a millimeter) in size. The shape of the particles suggests that they were melted in a high temperature event, such as an explosion. The iron may have oxidized (rusted) during the high temperature event or during the last 150 years’ exposure to environmental oxygen.
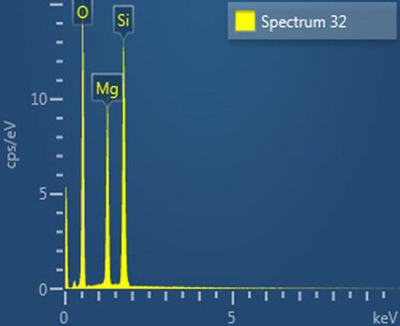
Particles of talc were also found. Talc is a mineral composed of hydrated magnesium silicate that was used as fire-proofing by some manufacturers of 19th century safes. Its X-ray spectrum shows prominent peaks due to magnesium, silicon, and oxygen.
Particles of mercury and particles of barite (barium sulfate) were also found. The significance of their presence is unknown. No direct evidence of gunpowder residue was found. But trace elements in the particles include sulfur and potassium, primary constituents of gunpowder, and lead, antimony, calcium, and barium, which are key parameters of modern gunshot residue analysis. Unfortunately, we do not know the exact composition of Civil War era gunpowder.
In summary, we have found three separate lines of evidence compatible with an origin in an explosion in a bank safe (iron oxide particles formed in a high temperature event, talc as a fire-proofing agent, and trace elements from gunpowder residue). These findings are somewhat speculative, and none are definitive; and, of course, we can not know how the notes may have become soiled or contaminated during the last 150 years. But we can say that our results are consistent with the robbery that was so well documented in 1866.
We have demonstrated that modern, non-destructive laboratory analysis can provide information about currency not available in any other way. A wide variety of other technological methods are available in addition to those we have used. Perhaps special applications may be found for the study of certain paper, ink, counterfeits, DNA, etc. Researchers need to include these possibilities in their thinking.
We would like to thank Patricia M. Anleitner (a former McCrone Associates senior research scientist) for her valuable assistance with this work.
Sources
The North Iowan, Osage, IA, May 31, 1866.
Treatise on Fire & Theft-proof Depositories and Locks and Keys, George Price, London, 1856.
Comments
add comment