Low Voltage Microanalysis
June 6, 2016
Presenter: Craig S. Schwandt, Ph.D., Director of Industrial Services and Senior Research Scientist, McCrone Associates
We’ll demonstrate just how good the new detector technology has become. 22 minutes.
Transcript
Charles Zona (CZ): Hello and welcome. My name is Charles Zona and I would like to thank everyone for attending today’s McCrone Group webinar.
Our presenter today is Craig Schwandt of McCrone Associates. Craig is going to tell us about low voltage microanalysis using the scanning electron microscope. Before we get started, I’ll give you a bit of Craig’s professional background: Craig is the director of industrial services and a senior research scientist with McCrone Associates and is also a co-instructor for Hooke College of Applied Sciences’ basic and advanced scanning electron microscopy courses. He specializes in X-ray microanalysis of particles using energy and wavelength dispersive spectrometry methods with the scanning electron microscope and electron microprobe.
Craig Schwandt (CS):
Thank you for the introduction Chuck. Hello to everyone who has tuned in for taking a few minutes to listen to some thoughts about conducting low voltage microanalysis with your scanning electron microscope and energy dispersive silicon drift detector. The technological advancements afforded by silicon drift detector technology are key to the successful application of low voltage microanalysis, these include both the large area of these detectors allowing for vastly improved counting efficiency, and their increased sensitivity for low energy X-rays.
One of the most important concepts to keep in mind before beginning any microanalysis is to think about the interaction volume produced by the electron beam of your SEM. Remember that the penetration depth of the interaction volume is a function of the beam energy and average atomic mass of the sample. This stylized interaction volume cartoon reminds us that the majority of characteristic X-rays originate from micrometer depths, especially for conventional microanalysis using beam energies of 15keV or greater.
With interaction volume in mind, the next consideration is the type of sample we wish to analyze, as well as what is our ultimate objective? Do we just want an idea about what something might be, or do we really need to quantify the results? If we want to quantify the results, how accurate do we want to be with these results? The beam energy we select will help us achieve correct answers to these questions. Do we want to know about particles? How does one mount those to avoid background interference? Does one have a thick flat polished sample, or is one interested in averaging lots of grains, or really quantifying the composition of a metal, or mineral, or perhaps one is interested in minor or trace compositions associated with biological materials?
In 1913 Henry Mosley published his empirical observations that the square roots of characteristic X-ray energy lines were proportional to atomic number. Along with Bohr’s model of the atom in 1914, the observations were responsible for ordering of the periodic table based on atomic number. Conventional microanalysis utilizes predominantly K lines, with some use of L-lines. For low voltage microanalysis we’ll be using more L lines than K lines.
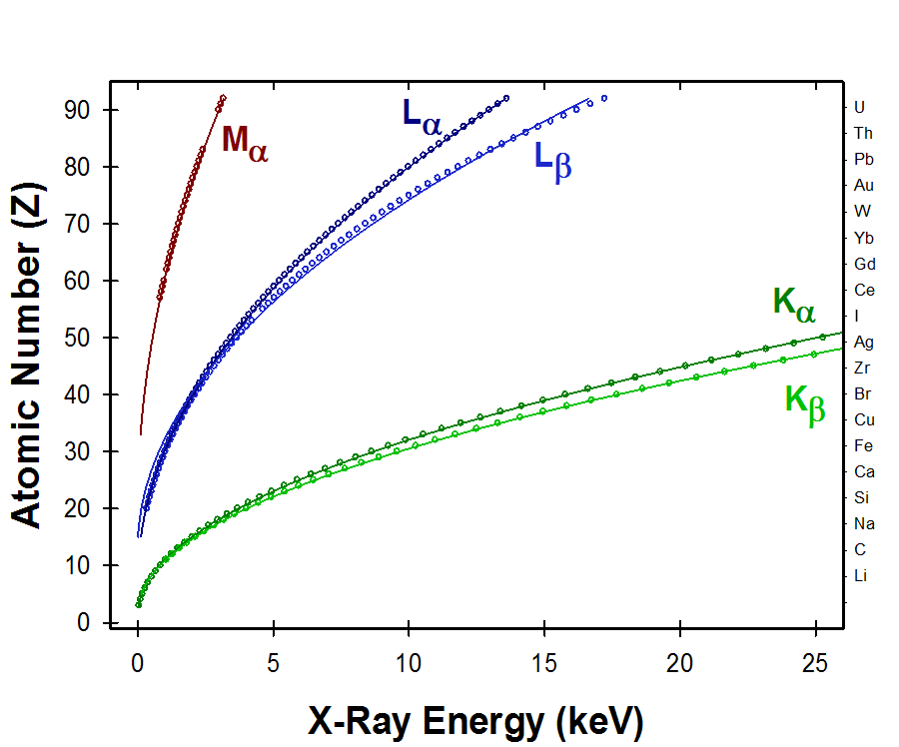
Hendrik Kramers described the distribution of X-rays produced when electron beams are hitting a solid target thus producing the continuum or bremsstrahlung radiation. As you can see from the red line (bottom right), ideally the intensity of low energy range X-rays should be very high, but in practice we observe this not to be the case, due to absorption effects. Absorption is another key factor to be concerned with, especially when conducting low energy microanalysis.

As an example of how successful low energy microanalysis can be, I will use a tourmaline example. Tourmaline is a hexagonal-trigonal cyclosilicate mineral containing boron. Borate anions are arranged in three-fold symmetry within the aluminosilicate framework. A number of cationic solid substitution possibilities yield a variety of colors to this popular gemstone mineral. The various coupled substitutions are named as end members with specific colors.
Conventional microanalysis utilized beam energies of at least 15 keV. Importantly, boron and oxygen were not measured. At beam energies of 10 keV and higher boron and oxygen suffer significant absorption effects. Thus it was either assumed there was little Si solid substitution and so analyses were normalized to six silicon atoms, and also assumed that boron was stoichiometric, so that oxygen was the difference of the analytical total from 100 wt%. Alternatively, one could assume the values were stoichiometric to 29 oxygen atoms and boron was the difference. Both methods rely on a major assumption and neither element was actually measured.
In my specific example, I had tourmaline crystals with chemical growth zonation that I wished to analyze. Here is a transmitted plane polarize light image of one example.
There are several factors to be concerned with when conducting low energy microanalysis. The first is whether one should use low vacuum conditions or carbon coat the sample and use high vacuum conditions. We must remember that low energy X-rays are greatly absorbed. The next consideration is to balance the over-voltage with the line selection for the elements of interest. You will likely want to experiment with this a little before actually deciding on the best energy to use. I’ll show the results of using internal standards, but for absolute best results one should conduct full measured standards quantitation. This approach however, often suffers for a lack of matrix matched standards, which are the ideal. Of course there are more peak interferences likely than in conventional microanalysis. Some factors specific to my example include conducting some experiments to establish if there are any crystal orientation effects associated with measuring boron. Also, is a spot or an area analysis better? As these may affect measurement and quantitation of heterogeneous materials.
With some experimentation, the first two concerns were established as the most crucial. A reasonable amount of testing was done to determine the best conditions for tourmaline.
Peak overlaps were considered to be of less concern, as peak deconvolution algorithms have become very robust. So, deconvolving the carbon from a low energy spectrum such as this provides a much better result than a situation where we have no idea about how much absorption is taking place and no way to correct for it.
In particular I’m talking about the use of low vacuum conditions. The conditions testing demonstrated significantly diminished boron intensity relative to high vacuum conditions. Thus, in the tourmaline case, the results was much better using carbon coating and high vacuum conditions than low vacuum conditions.
Using an Oxford Instruments AZtec system, I set up to use 4096 channels between 0 and 10 keV, and a process time of 5 for excellent energy resolution. I established that a good trade-off for boron intensity as well as calcium K-line production was an operating condition of 6 keV. I had great oxygen intensity as well as great boron intensity, though it doesn’t particularly look that way. Although the carbon intensity from the carbon coating is significant at least boron is measurable.
I used the established stoichiometric formula of tourmaline as published in An Introduction to the Rock Forming Minerals by Deer Howie and Zussman to calculate the composition in terms of weight percent and atomic percent. This is presented along the right side of the slide. The top left results are the quantitative results from AZtec using the Oxford Instruments Extended line standard set. The standards were simple oxides or in the case of boron, a boron nitride. I used some of the electron microprobe standards we have on hand at McCrone Associates to conduct a full measured standards-based quantitation and the results are almost indistinguishable. Importantly, though I also had to use a boron nitride as a boron standard. If I used a flat polished tourmaline as a standard, I’m confident that the result would be more accurate in terms of boron concentration. However, a wet chemical analysis method for measuring boron in tourmaline, which does not also use a meta-borate flux to get the tourmaline into solution doesn’t exist. Also, as boron has a high neutron cross section it is not well analyzed by instrumental neutron activation analysis. Other methods require a standard. Thus the best one can do in this type of situation is to characterize a sample very well and then use it as a standard, so that one would at least have an internally consistent set of data.
In the case of simple binary compounds, such as quartz the results can be very good. In this slide we see the results using the internal standards for beam energy conditions of 15 and 6 keV. The stoichiometric values are shown in the middle for comparison. In this case the matrix corrections although pretty good, are off at the lower beam energies.
However, if you have matrix matched standards with which to conduct full measured standards quantitation, then the results fantastic. The absorption, fluorescence, and atomic number corrections fall out and leave mostly sample preparation, detector performance relative to energy, carbon coating differences, and window corrections as the issues.
With more complicated compounds such as garnet, the results aren’t quite as good. The top result was completed using the supplied internal standards. The low voltage analyses utilized full measured binary oxide standards. The results are not too far afield. If we had a matrix matched garnet standard, I expect our low voltage analyses would be almost as good as the 15keV results.
It does depend on what material you examine though. In the case of steel alloys, especially stainless steels, the results of low voltage microanalysis are simply bad.
For materials that you have worked out the optimal conditions, you can even utilize low voltage conditions to conduct mapping. However, as with all elemental mapping of materials one needs to establish the appropriate dwell time for the lowest concentration element of interest or otherwise iteratively accumulate for sufficient peak intensity per pixel.
Low voltage microanalysis simply requires a little extra attention to details such as one would follow for electron probe microanalysis. First establish what you wish to find out, and then use your SDD to its full capability.
I hope you found this presentation useful. Thank you for your time.
CZ: Okay, thanks again for attending today’s webinar. If you have any questions, please go ahead and start typing them into the questions field and we’ll begin to answer them shortly.
CS: One question was, “Was there was any color change noticed on the stones after analysis?”
No. Not any. So, we are not burning these. There is much less opportunity for damaging materials when you use low voltage analysis than when you use high voltage probe current analysis.
CZ: Here is a question from Ed, “With carbon coating, is there a chance of boron contamination?”
CS: Pretty low. Most boron is not going to be in a quantity that you will really pick up in carbon coating —the amount applied with carbon coating. You might have some fractional percentage of boron in your carbon. But most of the sources, either carbon fiber or carbon rods for carbon evaporation and carbon sputtering are fairly pure so there is not likely to be a boron component in those.
CZ: Heather wants to know what detector do you use and from which manufacturer.
CS: In this case, this was an 80mm² X-max detector from Oxford Instruments. But, I could have equally have done this with any of the other vendors’ instrumentation.
CZ: So, it is not vendor specific. Great. Just in general, Eric wants to know the advantages of low voltage versus high voltage. A summary, maybe.
CS: In many cases, we will go back to the beginning where we discussed penetration depth. So, in general, you might want to use low voltage microanalysis to keep the analysis interaction volume very near the surface, so that you don’t penetrate deeply into the sample. In my particular case, with this example, I was interested in measuring boron and wanted to highlight the sensitivity, the low energy sensitivity of these new silicon drift detectors. But, otherwise, it is a combination. Do you need that light element sensitivity? You also get great response for things containing nitrogen much better than a sili or an older system. So, low energy microanalysis in terms of the elements you measure, and also keeping your interaction volume very near the surface, rather than penetrating deeper with a high energy microanalysis.
CZ: Tatiana wants to know, as it relates to pigments, a complex painting matrix with pigments of high atomic weight, such as lead mixed with laked pigments.
CS: In many cases, there are actually some M lines associated with those that actually are clear enough of other overlaps so that you could experiment with some low energy microanalysis, somewhere in the range of 4 – 6, or 7 keV. It may give you a much better result, especially if you used a sectioning method to isolate one of the pigments. If the painting was layered or something and you just wanted to isolate it to one pigment at a time, I think that the elemental spectra would actually be fine. There is really good response, and in many cases, quantitative results can come out just as well at the low energy because those M lines and the L lines in this region are very close to the oxygen in terms of the energy. So, the absorption corrections don’t need to be as extreme as if you were using high kV and using a high energy K line with the low oxygen energy line.
CZ: Okay. Ed is going back to your statement that low voltage microanalysis of stainless steels is bad, and he wants to know is that due to carbon dioxide absorption on the surface of the stainless steel?
CS: No, it is mostly due to the overlap of the critical constituent elements—the manganese, chromium, iron, nickel—the low energy L lines for those are very overlapped and it just doesn’t make for a very good mix. If you wanted to experiment with using the matrix match stainless standard you could perhaps improve it, but it won’t work out well if your standard set is with pure metals. Then the matrix corrections that will be applied are going to cause the quantitative result to be in error. So, this would be a good case to experiment a little on your own. If you have access to stainless steel standard materials, polish them flat, and then use those to take the measurements and use those as internal standards, and then compare your quantitative results. But, it is not due to the carbon dioxide.
CZ: I think that actually does it for the questions, Craig. We have gone through all of them. I would like to thank everybody again for attending today’s webinar.

Comments
add comment