Making the Transition to the ISO/IEC 17025: 2017 Standard (with Applicable cGMPs and cGLPs)
McCrone Associates, Inc. (MA) takes great pride in the strength of our Quality Management System and our ability to provide our clients with accurate and defensible analytical results. The Quality Management System at MA is based on the recognized International Standard ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories. MA has been accredited to this standard since 2014 and took the extra steps to include accreditation to cGMPs and cGLPs through A2LA, our accreditation agency (reference A2LA Certificate #3631.01). The culture of quality and staff pride in the MA brand, reputation, and services make management of the quality assurance programs fairly easy and straightforward. The quality processes have been embedded in everything we do and we will continue to elevate our quality program as we pursue accreditation to the revised ISO/IEC 17025:2017 in 2020.
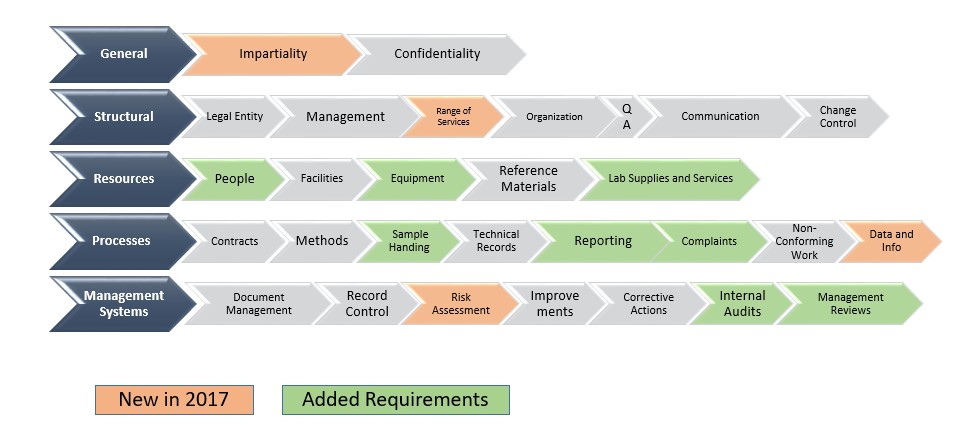
The revised ISO standard is reorganized, enhanced, and more aligned with the ISO 9001:2015 standard (Quality management systems — Requirements). The new standard is structured to build a lab from the ground up with added risk based processes. It starts with the general requirements of impartiality and confidentiality. Since these elements are integral to MA ways of working, we did not need any changes for this section of requirements. The structural requirements section covers the basic tenets required for a contract lab to do business. The lab must be a legal entity, have a defined management structure, and must define and document the range of activities for which it conforms to the standard.
Resource requirements are next. What resources are needed for the lab to perform its activities? There are new procedural requirements for supervision of personnel, and a new approach based on the demonstration of competency for personnel. Our personnel have always been our strongest asset and demonstration of competency was an easy transition for MA. Other resource requirements in this section of the standard include instrumentation, facilities/environment, metrological traceability, and externally provided products and services. MA enhanced policies and procedures and provided staff training to ensure that all requirements of the revised standard were met.

The next section covers process requirements. Process requirements include handling client requests, methods of analysis, sample handling, technical records, measurement uncertainty, ensuring the validity of results, non-conforming work, and reporting results. There are added requirements for sampling, reporting, complaint handling, and data management. Since the revised standard was published in late 2017, MA has been revising and improving existing processes to meet these new requirements.
The last section covers the management system for the lab. Management system documentation and control of documents, control of records, and actions to address risks and opportunities are covered in this section. Improvement, corrective actions, internal audits and management reviews are all covered. These areas have always been a strength for us since we first pursued accreditation in 2014 and required only minor changes to meet the new standard. MA has incorporated risk assessments in numerous processes such as change controls, internal audits, management reviews, etc. for years.
The new standard requires fewer written procedures, but the new A2LA checklist had over 70 new required elements. In addition to the primary checklist, MA is assessed against the cGMP and cGLP checklists which were also revised and updated. The processes, policies, and procedures are revised and implemented and we are ready for the assessment to the new ISO/IEC 17025:2017 standard to begin!
Comments
add comment