The Matrix Challenge: Picking Your Problem Cleanly
June 22, 2017
Presenter: Andrea Champagne, Research Chemist, McCrone Associates
Good analysis starts with good sample prep. Each different type of matrix presents its own unique challenges to particle isolation. This webinar discusses some of the specific challenges of different matrices, provides guidance and information for particle isolation, best practices for submitting samples, and discusses instances when the matrix should also be included with the sample. 25 minutes.
Transcript
Charles Zona: Hello. My name is Charles Zona, and I would like to welcome everyone to today’s McCrone Group webinar. Our presenter is Andrea Champagne of McCrone Associates Andrea is going to talk to us about good sample preparation and the challenges that different matrices present when trying to do particle isolation. The title of her talk is “The Matrix Challenge: Picking Your Problem Cleanly.” Before we get started, I will give you a bit of Andrea’s professional background.
Andrea is a research chemist at McCrone Associates, specializing in small particle analysis using light microscopy, micro-FTIR spectroscopy, and gas and liquid chromatography. Prior to joining McCrone Associates, Andrea worked as a method development chemist in the nutraceutical industry, developing methods to confirm the identity and purity of natural product raw ingredients. Andrea also spent time working as a forensic scientist, specializing in controlled substance analysis, fire debris analysis, and clandestine laboratory response. Andrea will field questions from the audience immediately following today’s presentation.
This webinar is being recorded and will be available on The McCrone Group website under the Webinars tab. Now, I will hand the program over Andrea.
Andrea Champagne (AC): Thank you so much. Good afternoon. Thank you for taking time from your schedules today to join us while we discuss some different matrices and the challenges they each present to contaminant analysis.
I am going to dive right in. What do we mean by the matrix? Well, we’re not talking about a special-effects-laden movie from the 90s; what we are talking about are the components of your sample other than the analytes of interest. In the instance of contaminant analysis, we are talking about the solution or powder that those little black specks have shown up in, also how your collection media can contribute and become part of your matrix. This happens quite a bit, but it is often overlooked. We are going to discuss that a little later on and I will give you some examples of when the way in which you are sampling your material should be considered as part of your matrix. Ultimately, you know what your matrix is; it is supposed to be there. The contaminants, which are not supposed to be there, so the matrix is the stuff you don’t care about. Except you really do care about it. And I am going to explain when and why.
Some of the most common matrices that we see here at The McCrone Group are powders, or tablets and capsules.
In the instance of a powder that has these little black specks, this is going to be fairly easy for us to isolate. We are going to examine that sample under a microscope, we are going to measure the particles, go in with a tungsten probe, and manually retrieve them. If they are big enough we can even retrieve them with a pair of forceps.
For a powder, like the one you can see on your screen, with nice, big, clean, crystalline morphology, not a lot of that is going to go with our particles, and we will be able to manually and physically isolate those. So, we don’t have to worry too much about the matrix in that case.
When we get into the finer powders, and the tablets and capsules, now we do have to be a lot more concerned about our matrix. We see a lot of tablets that have either discreet particles in them, where we can shave away some of that tablet material, or pull out a whole chunk of something. Other times, we see smears or stains on the surface. We are going to do the same thing. We are going to go in with our tungsten probe, we are going to remove the tablet material, and isolate just that stained portion. With fine powders, it is a lot harder to completely remove the matrix. In that case, we are going to go to a clean area of the tablet where there is some unstained powder. We are going to take that sample and prepare it for analysis as well.
The most common analyses that we do here (McCrone Associates) are micro-FTIR, as we mentioned earlier, or SEM/EDS: scanning electron microscopy with wavelength dispersive spectrometry. Those are our two main identification techniques. We want to be able to attribute any responses from those instruments that are to the matrix, and not the particle, appropriately, which is why we are always going to sample the matrix when we can.
Another very common matrix that we see here are liquids. This can be any type of liquid, from water to active pharmaceutical products, and anything in between. Once again, if we have a large enough particle, we will be able to go in and manually retrieve that with a tungsten probe or a pair of forceps. Sometimes, it will be easier for us to isolate the particles from the liquid solution by filtering. What we will do in that case, is, we will filter the solution through a sub-micron pore-size filter, we will rinse the container, we will rinse the filter body, examine it under a microscope, and we will retrieve our particles from the filter surface. This can be a lot easier to retrieve particles, but it can sometimes get you a little more than you bargained for. You might have been looking for this nice big chunk of gray material that looks like rubber stopper, and now we are seeing all of this amorphous stringy-looking particulate and small white pieces. We need to determine whether that came from the matrix itself, the liquid solution that we originally removed, or, perhaps, from the container that the liquid was put in. In these instances, we will always take a portion of the liquid and dry it down, so it can also be prepared for analysis afterwards to determine what should have been present: like, maybe that stringy particulate is protein residue, and what is truly foreign to the sample.
I am going to pause for just one minute. I have mentioned several times already that we do a lot of manual isolation. There is a reason that we like to do this. The whole process of visually examining those, physically picking the pieces out, this gives us some truly invaluable information. The appearance of the particulate, whether it is fine, crystalline, or a shiny metallic-appearing particle. Does it look like torn plastic? Are there cellular features that suggest to us that this came from a botanical source? All of this is going to be information we can use to inform our decision—our final conclusions—for results after the instrumental analysis.
The manual preparation of particles also gives us a lot of information about the texture of the sample. Is the black particle elastomeric? When I press on it, does it stretch out and then spring back like rubber or elastic? Does it just fall apart into a lot of little pieces? Is it really brittle? If it does fall apart, how much pressure do I have to put on it to get it to fall apart? All of this is going give me valuable information later on.
Silicone can take a dozen different appearances, and if I have an IR spectrum that shows me silicone in my sample, if it has an elastomeric texture, that is going to indicate to me that it is probably a silicone rubber. If it is smeared out like an oil or a grease, it is probably going to be silicone oil. This is why the manual manipulation of those samples can tell us so much.
Here at McCrone we are very fortunate. We have ISO Class 5 clean rooms with some amazing research microscopists, who are good enough that they can manually manipulate particles down to one micron in size, so, you don’t have to be able to see it with your naked eye in order to gain this valuable information. You just need to have the skills to manually move it.
Let’s talk about some specific matrices and the specific challenges that they present.
In this example, the question I am asking here is, we have little dark spots—are they adhered to the surface of my material or they actually embedded within? If they are adhered to the surface, they are just resting on the top of it, so the matrix is not interfering. The matrix is not going to become a concern for us. We are just going to be able to manually retrieve these particles, prepare them for analysis, and give you a firm conclusion.
If it is embedded, it is going to take a little bit more work. We are going to have to remove the matrix slowly and carefully. We are going to, in a lot of instances—the best way to describe it is shaving it off into small portions until we get just to the sample that we are interested in; we will remove that sample, clear away any matrix that is remaining on it, and then prepare the sample for analysis.
In this case, especially, the matrix needs to be prepared for analysis. If you have a dark spot embedded in nylon tubing, what you really need to do for your matrix analysis, there, is take a clean piece of nylon tubing and prepare it for analysis.
If you have something like food packaging, where something that looks very thin can have multiple layers—take, for instance, a Reese’s Peanut Butter cup wrapper, which is really thin, but actually has between six and seven layers of different polymers—you are going to need to take samples from multiple layers to determine whether or not the sample is truly foreign. It may be just a piece of top layer brown printing that got trapped in the middle. In a case like that, preparing your matrix is actually going to take longer than preparing your sample. You need to consider where it came from and all the potential sources of your matrix.
Another challenge can be aggregates. Aggregates are not a single discreet particle. They are more like a loose clump—for technical terminology—of multiple different things. You can see here, we have some discolored powders and shiny crystalline materials. There might even be little plastic or metal fragments buried in the middle of that. This aggregate itself is going to be multiple different types of particles. It can be different types of materials. And, sometimes—actually, very frequently—your matrix is part of that. Whatever material this aggregate was retrieved from also needs to be sampled and analyzed with the aggregate, so that we know which parts can be attributed to the matrix and which parts are truly foreign. Often times, too, you will see that maybe these brown areas are just melted or burned matrix. We need to consider the condition of the matrix at all stages.

I am going to talk for a minute now about what I like to call the lingering matrices. When I mentioned earlier filtering some liquids, there are certain liquids and components that just really like to stick around. When we fish a particle out of a liquid matrix, we are going to rinse it off and wash away any of that lingering liquid. Obviously, we don’t want it contributing too much.
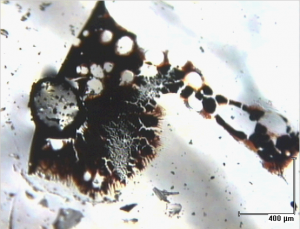
There are some materials, though—silicone, which I mentioned earlier, and protein, which we might find in cellular-based drug therapies—that really just like to hang out and stay on your particles. So, we wash these off with appropriate solvents. In the case of silicone, we will use hexane or nonane. You can see here from the image on the right just how much oil can be retrieved from a particle that looks like it was clean to start with.
If you get a particle that has been previously isolated from a liquid matrix, and you don’t have it available to dry down and examine, it is really important to find out that what that was and what it might be. Then you can determine the appropriate solvent to get rid of it. We like to start with water because we like to preserve it; we don’t want to risk dissolving our particle, but in the case of silicone, that is not going to take everything off. As much information as you can have about where a particle originated, the better off you are going to be, because it will guide what you are going to do to prepare it to give you the cleanest analysis possible.
Challenge #4 is when I start to talk about some of those introduced matrices. Swabs are a great sampling device. You can cover a whole big surface area with one swab; you can get into little tiny tubes and small pipe openings. If your swab surface picks up discreet particles, it is basically like looking at a filter surface. You can go in and you can manually retrieve those particles. You can size them and you can go on with your analysis.
If, instead of picking up discreet particles, you’re getting more of a stain or a residue—something that is soaking in to the fabric of that swab—you are going to have to approach that a little differently. In the case of the stained swab, what we are going to need to do is remove portions of the swab that are stained. If it is a fabric swab, or maybe nylon, we can cut out little discreet portions; or cotton swabs, like a Q-tip, we will tease out individual fibers. Then we will extract the stained areas in an appropriate solvent.
When you are dealing with true unknowns, an appropriate solvent can sometimes be multiple solvents, where you are looking at, perhaps, a range of polarities to make sure you are retrieving any possibilities. For each of those extracts, you also need to extract a clean portion of the swab in the same way.
Here we have layers of matrices. We want a clean swab that has not been exposed to anything. We want a swab that has been exposed to whatever the original matrix where you found these particles or stains were. For example, if you have a manufacturing vat that had sodium chloride solution and then you found a residue on the edges that you wanted to have analyzed, we would need a clean swab; we would need a swab that had been dipped in a sodium chloride solution, and then we would need a swab that picked up the residue. All of those are going to be treated in multiple ways, and each of those extracts is going to be submitted for analysis so that we can properly account for all that information: if it is genuinely from just the residue, or if it came from either of those matrices.
Another very popular sampling device is tape. Regular old adhesive tape is a great device for picking up small particles. When you have something small along the surface of, say, a plastic tube in a manufacturing facility that you want to sample, you grab a piece of tape, stick it on there, and peel it off. That is a pretty quick way to grab it, and you know it is not going to go anywhere.
Unfortunately, this also picks up some things that you are not expecting. If you are looking at the image on the right, I am assuming the particle of interest here is probably that nice long brown fiber; it looks, maybe, like a hair, but, we found a whole bunch of other things, too: this little red fiber, and a whitish one, and a black particle, and little white pieces. These are probably all common environmental debris; just dust, but we don’t know that when we look at the tape, and we have to determine which particle of interest is really there, and where the others may have come from.

Additionally, that tape then has to be brought or submitted to the laboratory for analysis. So you are either going to fold it over on itself and create a little tab, which is probably introducing some debris from the other side of the tape, as well, or you are going to stick it on a substrate, like a piece of paper, and you have any of the debris that may have been on the surface of the paper and those paper fibers all becoming part of the matrix now, which you need to be considering when you are isolating your particle.
It is not impossible, or necessarily all that difficult, to isolate a particle from the tape, but it adds some challenges. You have to know exactly which particle you are interested in, and you have to be aware of the amount of the adhesive. When we cut that out, we can see how much of the adhesive actually stays stuck to even a small piece of that particle. This can be removed manually, or with some solvent. Most adhesives are acrylate-based. It is very possible that in subsequent analysis you will see evidence of an acrylate. This would be reported as “we found this material, and it is an acrylate, possibly from the adhesive,” because we know it came from adhesive, but that might be erroneously excluding information if there is, in fact, an acrylate in your sample. Acrylic fibers, of course, are going to have acrylates in there, and certain other polymers as well, so you need to be aware of when the matrix is contributing information, and when, perhaps, assuming it is from the matrix is not helping you.
The final challenge that I will discuss are some of those satellite containers. We often look at something—it looks really clean, and that is fantastic—and that is how we are going to package our samples to be examined later. When you take a really close microscopic look at these, they are not as clean as you think they are. Plastic petri dishes are great to store things in, and if you look at those under a microscope, it kind of looks like it snowed inside. Every surface where the plastic bottom meets the top—every time it touches against each side, it is shaving off the microscopic particles; these run the risk of getting on a sample that is contained in the petri dish.
Likewise, any sample put in a petri dish could be shedding particulate into the dish itself, so, at the end, you don’t know if the particle stayed where you put it, if the particles from the petri dish wound up on your sample, or vice-versa. This can create a perfect storm of confusion for what you are looking for.
The other satellite containers that create a lot of issues are sterile containers:
- Sterile means that it has been treated to prevent microbial growth.
- Sterile does not mean particle-free.
As an example, I took this image from one of our sterile, 50 mL centrifuge tubes from the laboratory, and filled it about half-full with filtered DI water, gave it shake, and filtered it in our cleanroom. I found little particles and fibers. This is because the sterile container was not treated to minimize particulate. When we have an instance like this, and we report “here is all the particulate we found,” and someone says, “I was expecting fibers, not these little black things,” the first thing I always tell them is that it might have been from the container. If you tell me it is sterile, I am going to tell you that does not mean particle free. We will be able to identify what is in there, and the container itself is not necessarily contributing any chemical information, but we are not always going to be able to source the original location of this particle; if it came from the sample, or if it came from the container. In that instance, your analysis may be compromised by using sterile containers.
Now that I have explained all of these challenges and taken away all of your very favorite sampling devices, how are you supposed to approach this problem? Well, there are ways around everything, and sometimes—usually—knowing what has happened to a sample before we see it, or before it gets to whichever laboratory you are using, is the best defense. So, call us. Call on our expertise for how best to sample, or how to best package it. Email us and let us know “this is what we had to do, we did not have any other options for how we were going to sample this.” We will be more than happy to work with you to give you the best, most useful results possible, taking into account your matrix.
This is also the time to talk to your chemist, talk to your project leader, and find out which samples they may need as a matrix comparison. As we pointed out with the swabs, sometimes that may not be something you are automatically thinking of.
I want to thank everyone for their time and attention. I know we have a questions box on the screen. Please feel free to use it, and I will answer any questions you have.
CZ: Thanks, Andrea. That was interesting with the containers. As Andrea said, please go ahead and type in any questions that you might have, and we will go through them for a bit. I was going to ask, Andrea, based on your last slide, do you recommend to your clients that they should rinse out some of these containers before they use them?
AC: If it is possible, absolutely. We have the benefit here—in the example I showed—we have cleanroom water that has been filtered in a particle-free environment. Water carried through the laboratory may pick some stuff up, too, but, by all means, it can be very useful to rinse your containers out a little bit before you start putting your liquid samples in there. It is better than nothing.
CZ: Yes, at least it is a start. It helps.
AC: And in that case, send us a sample of your rinse water, as a matrix comparison.
CZ: We have a question here from Ann: “Are you usually rinsing the particulate coming from a liquid matrix?”
AC: Yes, we are, to the extent that we don’t have to be concerned that we are going to dissolve away our particulate. But when we filter, there is automatically a rinse with the clean room water that I have mentioned before. Frequently, if we know that those samples have come from a protein-based drug, we will go ahead, and as we are preparing them do individual particle rinses with an appropriate solvent, also.
CZ: Does anybody else have any questions that you would like Andrea to answer? Here is another one from Ann: “Are you performing the picking?”
AC: Sometimes, yes. The cleanroom is much better at it than I am, but we do see a variety. I am able to do some of my own preparation work. I will spend about half of my day with my face in a stereomicroscope, doing particle preparation, picking, including any of the rinsing necessary. Filtration has to be done in our cleanroom.
CZ: Laura asked a question: “Can you recommend a particle-free sampling device, such as loops, that are particle-free, or pipettes, perhaps?
AC: I wish I could. Honestly, I have found pretty much nothing that is truly particle-free. The best thing to do, and actually the approach that our cleanroom takes when we are doing filtering and the like, is to rinse those items that you are going to use with cleaned particle-free water in a controlled environment rather thoroughly before you do anything. That is truly the only way you are going to get something particle-free. Other than that, just out of packages, there is always going to be the risk of mild contamination.
CZ: Question from Mary Lee: “Do you have microtome capability for foreign matter embedded in a solid matrix?
AC: We do have microtome capability. We also, in some instances, have found that hand cross-sectioning is easier. That is something with response to an earlier question: am I particle picking now? No, I am not as skilled with that, but depending on the nature of the substrate, sometimes you can actually get a cleaner cut with a well-trained microscopist, but we do have a microtome, as well.
CZ: Is there an advantage to using in colored sampling devices to aid in differentiation between the sample and the sampling device?
AC: Color can help. What we tend to do, and use more to really make a particle stand out, are different lighting conditions. When we do our examinations, we look at our samples under oblique, transmitted, and coaxial illumination. We find a lot of times that one of those may be better, or maybe a combination of those may assist. Often, we use colors to improve our photomicrographs; we will put a strong contrasting color behind the particle to make it jump out. It helps. There are ways to work it if you don’t have that option.
CZ: Do you use gold-coated filters for IR reflectance from the filter surface, rather than transfer to sodium chloride window via a tungsten probe?
AC: When we do our IR, we will use the mode of infrared that is appropriate for the sample. When we do our particle prep, if it is going to be done in IR reflectance, we will put it on a reflective substrate. If it is something like a true fiber, which is 90% of the particles I think about as I’m thinking about it, we will do it by transmission, but that determination is what I would call more sample prep for analysis than necessarily isolating the particles.
CZ: I think that might actually do it for the questions. Thanks again for attending today’s McCrone Group webinar.







Comments
add comment