Is Your Product Stable in the Glass Packaging Selected for Use?
The Food and Drug Administration (FDA) has been and remains keenly interested in assuring drug products are stable and do not interact with their packaging. Hence, the required prescription for time stability testing of drug products—particularly injectables. USP guidance (USP 660 and 1660) on glass delamination, a visible marker for the reactivity between the drug product and the glass container, includes the analysis of the flakes and the interior vial surfaces; however, the specific analytical processes are vague and incomplete. The presence of glass flakes is a lagging indicator of vial deterioration, thus evaluation of the container surfaces during the drug development phases is crucial to avoid costly recalls. The flakes are sheets of glass, typically only tens of nanometers thick that shed from the interior glass surface when the drug product is excessively corrosive or reactive with the packaging selection. The flakes have a distinctive morphology but are very thin and the correct lighting conditions are needed to detect them. Under the right conditions the presence of many flakes becomes very prevalent due to many flakes of glass having broken away from the glass surface. As glass delamination has traditionally been defined as the presence of flakes, there is a perception that the presence or absence of flakes alone defines whether there is a stability issue or not. However, the presence or absence of flake alone, is an incomplete assessment of the stability of the drug product in the glass container selected. Despite glass packaging manufacturers providing a wide range of products and a wealth of information about the properties and durability of their products, time-stability studies using the actual drug product and container choice must be carried out.
Scientists at McCrone Associates have prepared and evaluated thousands of vials for glass delamination over the years. We have noted that due to the focus on flakes, some clients are unaware of the other indicators of product instability such as the pitting, corrosion, adsorption of flakes, and precipitation of particulate due to the changed composition of the drug product by incorporation of the glass.
As the real objective of each project is to determine whether there is potential drug product interaction with the selected glass packaging, a complete stability determination must also include assessment of the glass surfaces, not just the presence or absence of flakes. Corrosion of the glass containers is expressed as pitting of the interior surfaces, whether mild or extreme, and is evidence of potential drug product interaction. Pitting is a precursor to delamination of very thin layers of glass. Depending on the corrosivity of the drug product, delaminated flakes of glass may remain in solution long enough to be observed, or they may also dissolve into solution—a matter of corrosivity and time. Additionally, in some instances flakes are not compositionally consistent with glass, but are consistent with precipitation products due to the addition of the glass elemental components to the drug components. Thus, addressing product stability requires assessing the interiors of the packaging in addition to searching for flakes.
To provide our clients with highest level of confidence in our findings regarding their time-stability projects we have determined we must address all aspects. Therefore, McCrone Associates has honed a four-stage process to evaluate glass vials and ampoules for glass delamination, leveraging both the expertise of our world-class cleanroom scientists in microscopy and particle preparation and of our analytical scientists in SEM/EDS and XPS.
The process stages are:
- Observation of in-situ sample solution for the presence of glass delamination-like flakes, followed by sample filtration and examination of the filter using appropriate lighting conditions.
- SEM/EDS analysis, and sometimes XPS analysis of glass delamination-like flakes from PC filter, if observed.
- Optical examination of the vial interior glass surfaces for defects.
- SEM/EDS analysis of the vial interior glass surfaces for defects.
In Stage 1, the cleanroom scientist determines whether the vial contains particles that are morphologically consistent with glass delamination, examining each vial as received. Using a stereomicroscope, the scientist looks for free-floating particles having morphologies consistent with glass delamination. Regardless of whether particles are observed in the initial examination, the scientist then filters the contents of the vial onto a suitable polycarbonate filter. The scientist examines the filter, first using a stereomicroscope and then under a polarized light microscope (PLM) at higher magnifications. The scientist captures digital photomicrographs of any glass delamination-like flakes on the filter.
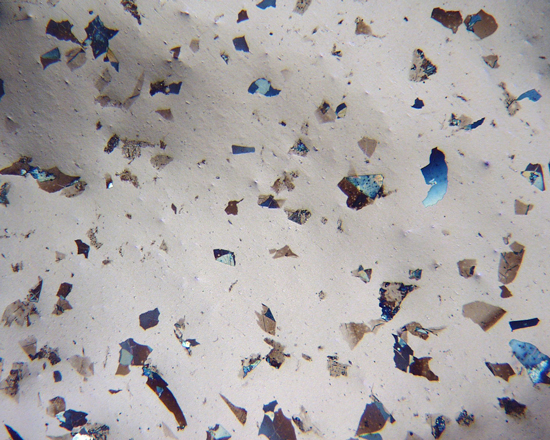
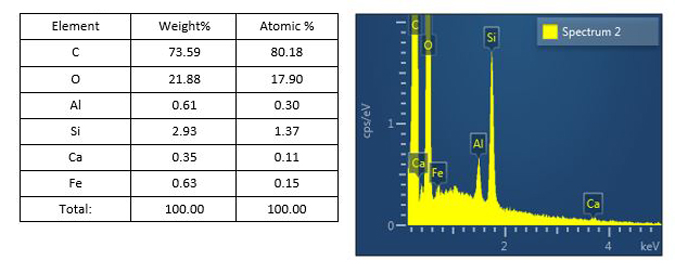
The chemical composition of any glass delamination-like flakes found on the filter is determined in Stage 2. The cleanroom scientist prepares representative flake-like particles for analysis by SEM/EDS. The particle preparations are analyzed, and SEM images of the flake-like particles are captured. The same particle preparations can be analyzed by XPS if the flakes are too thin for meaningful EDS analysis.
In Stage 3, the cleanroom scientist examines the interior surfaces of the vial for evidence of surface defects including glass delamination and pits. To access the vial interior, the scientist breaks the vial in a carefully controlled manner, maintaining the spatial location of the glass pieces. The interior surfaces of the vial are examined and digitally imaged under a stereomicroscope, followed by a PLM examination and imaging at magnifications as great as 400X to 500X, if needed.
Stage 4 involves the examination of broken vial pieces from the neck, body, and heel, which a cleanroom scientist mounts for SEM imaging. The aim of the analyst is to examine and resolve at high magnifications the interior surface of the broken vial glass pieces, documenting the presence or absence of glass delamination debris or other surface defects.

Ideally, samples for analysis are accompanied by empty retain glass containers as control samples so that optical and SEM inspection can assure the integrity of the findings. They provide a necessary baseline condition, because occasionally processed empty vials are pitted before filling. With this four-stage process McCrone Associates provides our clients with the highest fidelity independent findings regarding their time-stability testing samples in the packaging selected, as received.
If your company is experiencing issues with glass vials or other glass packaging containers, please contact McCrone Associates to speak with a glass delamination subject matter expert scientist.
Comments
add comment