Solving Pharmaceutical Production Problems With Investigational Analysis
Pharmaceutical clients often refer to unknown particulate materials that occur in products as contaminant analysis or analysis of foreign particulate matter. These particles are microscopic evidence of undesired or unexpected problems occurring during a production process. The FDA requires that these contamination problems be investigated and resolved in a timely fashion. McCrone Associates’ ability to identify microscopic-size particulate contamination is often essential to resolving product problems.
Common contaminants in pharmaceuticals include natural and synthetic fibers, silicone, plastics, rubber, metal particles and corrosion products, glass particles and delamination flakes, skin flakes, char particles, detergents, lubricant oils, fluoropolymers, and graphite. The contaminants tend to occur as heterogeneities or particles: discrete, highly-concentrated accumulations of the contaminant. McCrone Associates has developed specialized methods, which we call “the particle approach,” to detect and analyze these contaminants and many others.
The particle approach to contaminant analysis of both traditional and biologic-based pharmaceuticals includes gathering of background information concerning the sample, sample examination, isolation of the contaminant particles, and microanalysis. Analytical methods often utilized include one or more of the following: polarized light microscopy (PLM), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy imaging with energy dispersive x-ray spectrometry (SEM-EDS).
The key to developing and executing a successful analysis plan is working closely with our clients to understand their product and production environment. This understanding allows us to efficiently separate the contaminant from the product material for analysis.
Common Contaminant Issues
Fibers are a commonly encountered contaminant. Fibers can be manually isolated for analysis without the dilution effect from the product material. Fibers are usually identified using FTIR to confirm their chemical identification and PLM is used to provide greater detail about their sources. For example, many fibers may be identified compositionally by FTIR as cellulose, however, with PLM, the finding can be refined and more specifically established as cardboard, paper, cotton, linen, or rayon. Sometimes, a variety of fibers is found within the same sample, and PLM is an efficient method of sorting out the various types.

Glass is another contaminant that is often observed during quality control screening. Glass particles can be generated by from external sources such as other vials, glassware and lighting, from internal fracture of the vial, or from delamination of the inner vial surface. Because of their density, glass particles often sink rapidly to the bottom of filled vial. Glass particles may be recovered by direct sampling or by filtration.
Glass delamination flakes are extremely thin and may be missed during visual examination. If a larger number are present, a “twinkling” effect in the solution is observed, and is best observed using a fiber optic light source. Glass delamination usually occurs when a highly acidic, highly basic, or sodium chloride solution is stored in an incompatible container.
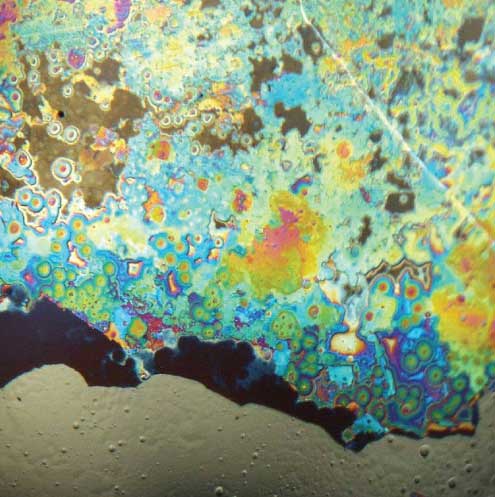
Tablet contamination occurs in a variety of ways. Sometimes tablets are known to contain metal contaminants because they fail the magnetometer screening on the production line. For these tablets, the contaminant is typically a relatively large particle (>500 µm). Analysis of these particles is quickly accomplished with SEM-EDS. Tiny metal particles (wear or corrosion particles) that are mixed with tablet material can cause gray or black spots, or surface discoloration. Tan or brown particles on or in tablets are frequently found to be thermally degraded (charred) excipient material.
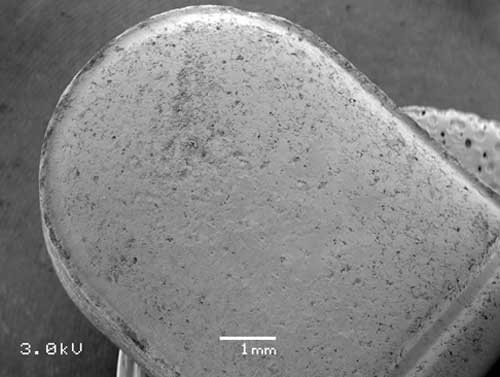

In other situations, discoloration on tablets can arise as a result of ingredient mixing heterogeneities, or incomplete coating applications. Recent improvements to confocal Raman microspectroscopy allow mapping of tablet components, which can address these types of discoloration.
Successful analysis of particulate contamination is essential to understanding many production problems in the pharmaceutical industry. Our staff of expert scientific detectives are ready for your next investigational challenge — contact us.
Comments
add comment