Splitting Hairs (and Fibers): 4 Cross-sectioning Methods
May 6, 2020
Presented by Sandra Koch, Ph.D., Senior Research Microscopist, McCrone Associates.
For morphological analysis of diverse samples, it is extremely useful to become proficient in cross-sectioning techniques. This webinar will discuss sectioning hairs and fibers, but the techniques demonstrated can be applied to other materials. Being able to directly observe the internal structures of a hair or the cross-sectional shape of a fiber can provide useful information for sample identification and differentiation. This webinar presents an overview of four methods that can be used to obtain information on the cross-sectional shape of a material and discusses the benefits and limitations to each method, with a special focus on ease of sample preparation and the analyses that can be reliably conducted with each method.
TRANSCRIPT
Charles Zona: Good afternoon, and welcome to another McCrone Group webinar. My name is Charles Zona, and today, we welcome back Sandy Koch. Sandy is going to talk to us about preparing hairs and fibers for cross-sectioning. Her presentation is titled “Splitting Hairs (and Fibers): 4 Methods of Cross-sectioning.” But before we get started, I would like to give you a bit of Sandy’s background.
Before joining The McCrone Group, Sandy worked for the FBI for over 16 years as a trace evidence examiner. She has taught numerous workshops and short courses on crime scene evidence collection and trace evidence analysis. Sandy is a Fellow of the American Academy of Forensic Sciences, and a founding member of the American Society of Trace Evidence Examiners. She spent five years researching variations of human hair microstructure at Pennsylvania State University, where she earned her Ph.D. in Biological Anthropology. Sandy has published papers on topics such as the forensic analysis of hairs, fibers, fabric damage, and feather identification. She also teaches the Forensic Hair Analysis course here at Hooke College of Applied Sciences, a member of The McCrone Group.
Today’s webinar is a little bit different in that it is a recording, and not live. However, you can still ask questions by typing them into the questions box. We will answer all of your questions individually in the coming days. And as always, this webinar will be available as a recording on The McCrone Group website under the Webinars tab. And now, I will hand the program over to Sandy.
Sandy Koch: Thank you Chuck. Today I am going to share a few methods that I find useful to section hairs and fibers to better observe their cross-sectional shape and internal structures.
A cross-section is made by cutting across a material at right angle to its main axis. We can use physical cross-sections to more clearly observe the shape of a fibrous material and I’ll be sharing a few methods throughout this webinar. Today I am focusing on hairs and fibers, but you could use these methods for other materials just as easily. The main concern for any cross-section method, is that you are careful to not alter the shape of the material that you are trying to analyze, especially if you are hoping to get accurate measurements.
The four methods that I am going to discuss are how to use a hot plate and polyethylene to embed materials for cross-sectioning, I’ll demonstrate how to use cross-section plates, an ultramicrotome for ultrathin sections, and how to visualize the cross-sectional shape of a fiber by optical sectioning—so I’m presenting three physical sectioning techniques and one optical.
Polyethylene Sheets and a Hot Plate
For the first method, you can use a disposable pipette or sheets of polyethylene film. You will also need a hot plate, glass microscope slides, single edged razor blades, and double sided tape.
Here I am using a polyethylene pipette. I thread the hairs down the tip of the pipette and cut to length, or you could cut the pipette prior to placing the hairs inside. The pipette tip is placed on a slide, and that slide is set toward the edge of a hot plate. I then place another slide on top of this, slightly offset, at an angle, so it will be easier to separate later, once the polyethylene has melted. Here, I am holding onto the edge of the slide and using a tool to press down on the slide, and to press down on the polyethylene to make sure it melts with as few air bubbles as possible, and really surrounds that hair. You can start to see the change in the polyethylene as it melts around the hair and embeds the material that you’re going to want to section.
You can use the same method with polyethylene sheets. Here I have placed several fibers from a sample of carpeting on top of one sheet of polyethylene that was cut to fit within the width of a slide, and I placed another sheet on top of that, and I try to align the fibers parallel to each other up as best as I can…here.
And then I’ll place another glass microscope slide on top, slightly offset, place it on a hot plate until the polyethylene has bonded together, embedding the fibers within, basically, a sandwich of polyethylene.
After I let the sample of fibers embedded in polyethylene cool, I’ll peel it off of the slide, and then I’ll use a single-edged razor blade to slice sections, as you can see here.
And then those sections are turned on their side, and you can focus on those with a stereomicroscope to start to see the cross-sectional shape of the fibers.
I found that placing the section on double-sided tape allows me to hold the sections upright and in position so that I can examine them better under a microscope, and really see the cross-sectional shape.
The benefits to using the polyethylene method for cutting sections are that it is fairly quick and easy, and most labs will have these supplies on hand. Some drawbacks may be that it can be hard to orient the samples at exactly 90° to the main axis, and when you are embedding and sectioning by hand you may alter the shape of the material, so you might not be able to get accurate measurements.
Fiber Cross-sectioning Plates
The next method that I want to show you is how to use cross-section plates. These are really useful because they double as a slide to place on your microscope stage and you can examine the cross sections directly.
The first thing to do is cut a length of thread, and thread both ends through one of the holes, creating a loop. You’re going to then place the material of interest, whether it’s a group of fibers or a single fiber, and add some extra packing material, ideally of a different color so you know where the material of interest is—place that within the loop. And the packing material is really to fill in the space in the hole in the cross-section plate and help hold your fibers in place. Youre going to pull the thread to tighten the loop and tighten the fibers of interest up against the plate, so that when you draw it through, you’ll have your fibers there.
You want to pull the material slowly through the hole, but not too far, just enough so that you have a loop extending through the hole. You’re going to turn the plate over, and using a razor blade, slice off the excess on the back side. Now, if you have dull blade, it may take a litte bit more time, so you may want to have a good supply of razor blades. After you’ve used one for one section, then get a new blade for the next section.
And this shows why you need the packing material. There is some manipulation around the fibers here, so you want it well held in place.
Once you’ve trimmed the material, for the most part, you coat it with clear nail polish, and this will hold the fibers in place when you start to cut the next side, and then let it dry.
Once the back side nail polish has dried, you can turn the plate over and carefully slice off the remaining loop of fibers. So this is the point where you want it well held in place by the nail polish and the packing material, so that when you use the slide…just slice off so that the fibers are held in place. You may have to trim a bit more to get the cross-sections to be even with the surface of the cross-sectioning plate, but this will take a little bit of practice.
Again, coat this side with clear nail polish. Your cross-sections are now firmly embedded in the slide and preserved, so you are now ready to use this slide to examine the sections under higher magnification with a light microscope.
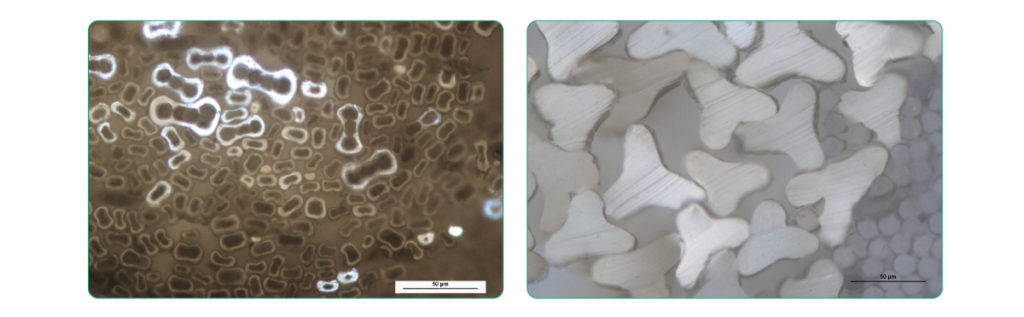
These are examples of fiber cross-sections when viewed at higher magnification with polarized light microscopy.
And these are some hair samples: human hair on the left, and non-human animal fur on the right.
The benefits of using cross-section plates are that once you have cut the sections, the material is ready for analysis using a microscope, and the nail polish serves as the embedding material to preserve the cut sections.
Some limitations that you should be aware of relate to how much packing material is used. If too much is added, you may not be able to pull the thread through the hole, but if too little is used, the sections may not stay in place. The amount of tension placed on the thread and from the packing material as you pull the material through the hole in the plate can also cause some materials to deform, so it may take some practicing to become proficient at sectioning with this method.
Using an Ultramicrotome
The next method I have used to cut sections is with an ultramicrotome. This requires more specialized equipment: an ultramicrotome, a glass or diamond boat to cut the sections, resin and molds to hold the samples, and an oven to cure the resin.
Before sectioning with an ultramicrotome, the sample has to be oriented perpendicular to the cutting blade, so there is a bit of time needed for setting up of the sample prior to sectioning. The boat with a diamond blade at its end is filled with water, and as the sections are cut, they float on the surface.
With these ultra-thin sections, I found that some tended to wrinkle, but by waving a cotton swab that had been dipped in chloroform over the sections that were floating on the water, the chloroform exposure helped the sections to flatten out. Then staining the sections helps to reveal the structures of interest and I have images captured using oil immersion microscopy and transmission electron microscopy.
The benefits of using an ultramicrotome is that you can set the thickness of the sections to be cut, and there is less sample distortion, so there is greater accuracy in any measurements that are taken. The limitations are going to be related to amount of sample prep and equipment that are needed.
Optically Sectioning a Material
The next sectioning method I want to discuss is one where you optically section a material instead of physically cutting across its length. Using a compound microscope and samples that are longitudinally mounted, you can figure out the cross-sectional shape of a fiber by moving the stage up and down and focusing on the different planes of the fiber.
Here I have a trilobal carpet fiber that you can see the different planes of the fiber coming into focus and going out of focus. And you can see that the process reveals that the fiber is a trilobal fiber.
You can train your eye to recognize different fiber forms by using known samples that you already have cross-section images of, either from having made physical cross-sections, or if you have SEM images of the fiber ends, and then when you examine samples of those same fibers in longitudinal mounts and compare it to what you know is the cross-sectional shape, you will become familiar with the different features that help you determine cross-sectional shape optically.
Conclusion
Overall, physically cross-sectioning a hair or fiber will reveal much of the sample morphology, but the embedding methods differ in how the samples may be packed, oriented, and how they may be deformed by the act of sectioning. The choice of sectioning method will depend on what equipment you have access to, the time you have to do the analysis, your sample size, and what information you need, such as do you need precise measurements or just information on the size range and sample shape.
Thank you so much for your attention today and I hope you try out one or two of these methods next time you need to determine the cross-sectional shape of a hair or fiber. If you have any questions, please email us, and I will get back to you as soon as possible. Thank you.
Charles Zona: I’d like to thank Sandy for doing this presentation today, and all of those out there who joined us and tuned in. We really appreciate it. Please check out our Webinars page for upcoming McCrone Group webinars. Thank you.
Comments
add comment