Understanding Surface Properties Using XPS
April 4, 2019
Chemical reactions between solids and their environment are governed by the identities and the chemical states of the molecules found on their surfaces. In order to predict how a material will react—or understand how it has reacted—to a given chemical environment, surface-sensitive analytical techniques must be utilized. One such technique is X-ray photoelectron spectroscopy (XPS), also known as electron spectroscopy for chemical analysis (ESCA). XPS exploits the nanometer-scale path length of electron photemission in solids to reveal information about a sample’s surface composition and chemistry. This free webinar provides a brief overview of the underlying theory of XPS, and demonstrates a variety of XPS analysis techniques that can be performed, including compositional depth profiling and chemical state characterization, using our newly-acquired ThermoFisher Scientific NEXSA instrument.
(Note: due to animations within the presentation, some images and captions are not visible in the PDF format; you may view the animations in the video.
Transcript
Charles Zona (CZ): Hello, and welcome to another McCrone Group webinar. My name is Charles Zona, and today we are happy to welcome Doug Meier. Doug is going to talk to us about X-ray photoelectron spectroscopy, otherwise known as XPS. Before we get started I would like to give you a bit of Doug’s background. Doug is a senior research scientist with McCrone Associates. He specializes in surface sensitive spectroscopies, such as Auger electron, X-ray photoelectron, infrared reflection absorption, thermal desorption, and low-energy electron diffraction. Doug was awarded the U.S. Department of Commerce’s Silver Medal for his work in the development of conductometric chemical microsensor array technology for the detection of chemical warfare agents. He also has over ten years of micro beam analysis experience prior to joining McCrone Associates.
Doug will field questions from the audience immediately following today’s presentation, and this webinar is being recorded and will be available on The McCrone Group website under the webinars tab. Now I will hand the program over to Doug.
Doug Meier (DM): Thank you for the introduction Charles, and thank you to everyone joining us today for our webinar “Understanding Surface Properties Using XPS”; X-ray photoelectron spectroscopy. I’m going to talk with you today about how XPS can be a valuable addition to your analytical toolkit by helping you to characterize the surface properties of your samples. First, we’ll discuss what is meant by the term “surface,” and why it matters. After that, we’re going to describe how XPS is used to acquire the surface sensitive data you require. Following that, we’ll show some examples using some everyday materials to show what XPS can extract in terms of information from that material, after which, we’ll describe the value of ion beam depth profiling for this type of experiment.
So, what do we mean by the term “surface?” For our purposes, the surface contains the atoms and molecules on the exterior of an object that can interact with the energy atoms and molecules outside of that object. For different depths into an object, however, surface can mean different things depending upon what is being interacted with. For example, there is very little outside of the object that makes it in and performs any chemistry at greater than 1,000 nanometers. At depths beyond a thousand nanometers, we generally refer to this region as the bulk of said object.
However, certain things, such as light, can penetrate some depth into a material and perform chemistry or have other interactions with this near-surface region material—less than 1000 nanometers. The atoms at the exterior, this top surface layer shown in green, however, is the first thing that an impinging atom or molecule interacts with when it comes into the object. These surface atoms are the atoms whose properties we need to analyze in order to understand many of the characteristics of materials that we might find in our laboratories.
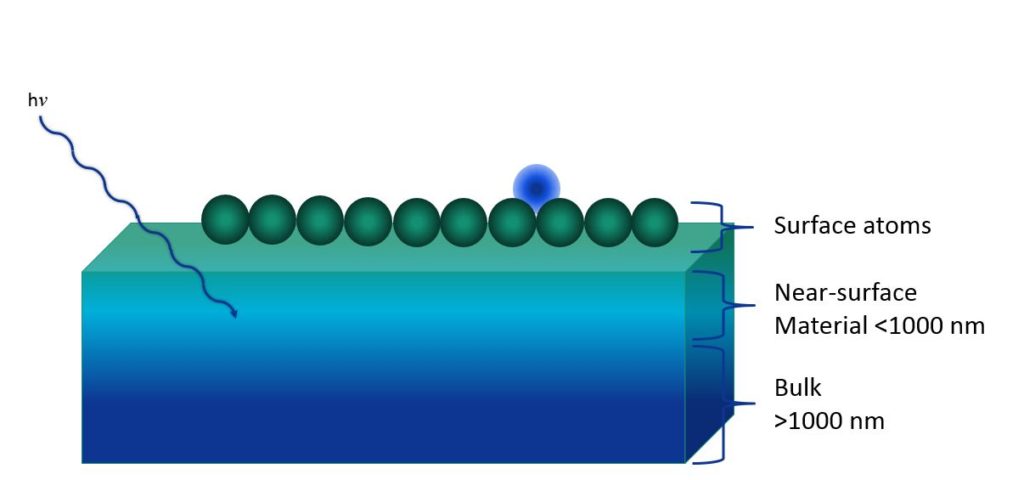
Many properties that we’re interested in for our materials are specific to surfaces. Depending upon what problems we’re interested in solving, a surface property can be governed by material that appears at different depths, for example, corrosion properties, and wear, and the appearance of an object. Is it shiny? Is it dull? What color is it? This information comes from the first hundred or so atomic layers, or more, within a sample. However, oxidation properties, passivation, and tribology are affected to a greater degree by these atoms that are closer to the surface, but are not necessarily the very exterior of the object, say, 10 atomic layers or so. Finally, properties such as adhesion, wetting, and heterogeneous catalysis are strongly governed by the identities and the oxidation states and the bonding of those atoms in the surface layer, or the < 10 atomic layers of the surface.
Now that we’ve discussed what we mean by surfaces, let’s move on to describe how XPS acquires the surface-sensitive data that we’re interested in acquiring. XPS works via the photoelectric effect, in which a quantum of light or a photon—in this case, an X-ray—is absorbed by a bound electron in the atom. This bound electron is ejected, and the energy of that electron can give us information on the atom from which it came. This effect was first experimentally observed by Heinrich Hertz in 1887, in which it was found that the voltage required to jump a spark between two metal plates was lower when light was shining on the plates than when light was not shining on the plates. This effect was successfully explained by Albert Einstein in 1905, earning him the 1921 Nobel Prize in Physics. The practical applications of this effect are wide reaching and touch every aspect of our lives, from photocopiers to solar panels. This effect is also responsible for providing the elemental information and the chemical state information, as well as the depth resolution, achievable via XPS.

I’ll show you next how it is that it accounts for the depth resolution of XPS. This graph shows why XPS is so surface sensitive, because we’re measuring the energy of electrons that have been ejected from atoms via the photoelectric effect. This graph of electron attenuation length in solids versus electron energy, also sometimes referred to as the universal curve, or also the inelastic mean free path of electrons in solids, shows us how far an electron goes through the solid before it interacts with another electron. When an electron interacts with another electron in a solid, energy is exchanged, and when the energy is exchanged, the information is lost. So essentially, the attenuation length tells you how deep from the surface we can expect for an electron to escape without having interacted with anything else—that is, without losing the energy that is necessary for us to maintain information from the atom from which it came. Now, in doing XPS, we’re typically using X-rays from the aluminum K-alpha line to excite our sample and to interrogate our sample. That X-ray has an energy of 1,487 volts. Thus, any ejected electron must have some energy equal to or less than 1487 electron volts. Well, if we trace over to the attenuation length, we find that the preponderance of electron energies have an attenuation length less than two nanometers, which is to say that any electron produced by the photoelectric effect in this solid deeper than two nanometers has a greater likelihood of interacting with another electron and losing its information than one that is at less than two nanometers. Thus, any of the photoelectrons that we measure that are of characteristic energies for the atoms of interest must come from this region that’s around two nanomete rs or less. That means that our information depth is approximately 10 atoms thick in XPS, and that is what gives us our surface sensitivity.
Shown here is the ThermoFisher Scientific NEXSA instrument. It is the instrument that we use at McCrone Associates to acquire XPS data. The schematic to the right shows how this instrument works. Essentially, you start at the X-ray source, which is an electron beam impinging on an aluminum target to produce aluminum X-rays. Those aluminum X-rays are scattered through a monochromator crystal in order to separate all of the X rays other than the X-rays that we want to use for our probe, which are the aluminum K-alpha X-rays discussed in the previous slide. Those X-rays are directed onto the sample in order to generate the photoelectric effect at the sample, producing the photoelectrons that we will measure. Also impinging upon the sample is an electron flood from the flood gun. The electron flood from the flood gun prevents sample charging as the X-rays cause electrons to be emitted from the sample. If the sample is non-conductive, this can change the position of peaks in our spectra and make analysis difficult for non-conducting specimens. Also impinging upon the sample is the ion gun. The ion gun allows for us to selectively remove layers of material and to get information in three-dimensional space so that we can analyze material at the surface and then see what is next underneath, and so on, into the depth of the sample. The photoelectrons are emitted through lenses into the hemispherical analyzer, which separates out the electrons of a given narrow band-pass, or energy, and those are then collected by the detector. The hemispherical analyzer is scanned through the entire energy scale, or some segment of it, and then the spectrum is generated at the detector.


Now that we’ve explained how XPS exploits the photoelectric effect and the short attenuation length of photo-emitted electrons to acquire surface sensitive data, what information can we extract from this data about our samples?
The next few slides we will show examples from common materials of information that can be extracted from a sample using XPS. As we touched upon previously, XPS is used to extract information about atoms on the surface by measuring the energies of photo-ejected electrons. This is shown in equation form here:
BE = hν – KE
The kinetic energy, or KE, is what we measure. hν, or the energy of the photon or X-ray that created the electron hole, indicates the energy that went into the system. The difference between the two, then, is the binding energy, or the strength of the bond that held the electron into its orbital around the nucleus. This photoelectron binding energy in which we’re interested depends upon the identity of the element and the electron shell from which the electron came. Electrons are ejected from discrete atomic orbitals, the energies of which are governed by the number of protons in the nucleus. Slight changes in the position of any given peak can give you information about an element’s chemical state. The changes in the electron density of the valence orbitals actually changes nuclear shielding on inner shell electron energies. It’s one of the benefits of quantum mechanics, an electron in an orbital further away from the nucleus can cause shielding for an electron closer to the nucleus.
Now we’re going to see how this works on a real-world sample set. Here, I show two pieces of galvanized plate. I recently had to have my furnace replaced at my house, so the HVAC system had to be changed because the furnace was a new size. So, I have pieces of the old plate, shown in the image on the left-hand side, and pieces of the new plate that they used to put in the new furnace and get it to fit with the rest of the house. Now, these are both supposed to be galvanized plates, however, they both look very different. You can see that the old plate has much larger crystal domains visible, than the much more glittery and small crystal domain new plate does. We can take these two samples and put them into a scanning electron microscope in order to do quantitative analysis by EDS, and see that their spectra are really quite similar, even though visually they look quite different.


The quantitative analysis of these also is quite similar. The most that we might be able to say from these is that the older piece of plate is perhaps a bit dirtier having been in the environment for a few decades longer than that newer plate had been. However, we can see that the zinc amounts are very high, we can see a little bit of iron, perhaps, the steel underneath. We can see a little bit of chromium in both, less than one percent, also, perhaps, from the steel. Both seem to have roughly the same amount of phosphorus, and they look, by EDS, quite similar.
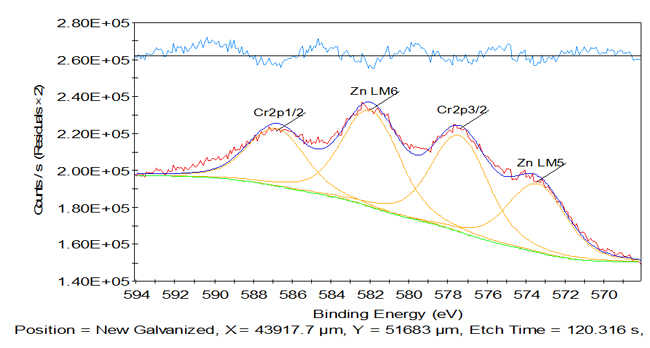
Here we see the XPS spectra overlaid, on the right, for the old galvanized plate, in red, and the new galvanized plate, in green. This is a survey of the entire spectrum and it looks quite busy, but we can zoom in on sections of these spectra in order to identify differences between the two spectra. Let’s zoom in on the section between 450 volts and 720 volts. Here we can see on this surface that there are actually recognizable differences between these two plates on the near-surface scale. We can see, for example, that much more zinc is visible on the old galvanized plate surface than there is on the new galvanized plate surface. Those red peaks are considerably stronger intensity than the green peaks for the new galvanized plate. On the other hand, in the new galvanized plate we can actually identify the chromium peak; whereas, in the old galvanized plate it is unsure whether there is a chromium peak there at all. Here in the center, we note on the new galvanized plate that we have a considerably larger chromium peak than the old galvanized plate. However, we do also have some zinc peaks in here that may result in some interferences. How can we sort this out?
As it happens, peak fitting in XPS is a very mature method for sorting out complex spectra. Background subtraction and peak fitting from a high resolution region of interest, such as the chromium 2p lines shown here, can be used to help you resolve peak overlaps, in this case, with some of the zinc Auger lines that sneak in up behind the chromium lines. In this way, we are able to take a complex spectrum and still sort out the information we need, even when overlaps might challenge us.
Here’s another example of a common item that one might measure in an XPS. This is high-density polyethylene, or what you’re more likely to see it as is milk jug plastic. This is a piece of the milk jug that I took apart from home and cleaned up so that we could demonstrate XPS of a non conductive material, in this case, a polymer. We can also show chemical state information, as well, with this. What you’re seeing on the red trace is a spectrum of the milk jug plastic in the carbon region, on the left, and in the oxygen region of the spectrum, on the right, that is as received—essentially, taking the milk jug plastic, cut a piece out, cleaned it, and placed it in the spectrometer. You can see that we have one simple peak for carbon, which is the carbon-carbon bond and the carbon-hydrogen bond that one expects for polyethylene, and a small amount of oxygen on the right-hand side. However, it doesn’t take much to change this spectrum. I took this piece of milk jug plastic and placed it into a plasma cleaner for about a minute or two. You take this out and put it in the XPS; it visually doesn’t look any different, but the spectrum changes dramatically. We can see that we are now getting a much larger multi-peak feature that shows that our carbon carbon bonds at the surface have been replaced with a large number of carbon-oxygen and carbon double-bonded to oxygen species, and concomitantly, we can see on the right-hand side, that the oxygen intensity has increased substantially on the plasma-cleaner-treated plastic than on the plastic before treatment.
We’re not necessarily limited to macroscopic samples. One of the sample holders that came with our NEXSA is a zero background sample holder for fibers. It essentially has an open space in it that allows you to stretch a fiber across the open space and get the spectrum only of the fiber. So, we’re going to try this on a fiber. Here’s a piece of carpet fiber. We discovered in taking an infrared spectrum of this fiber that it is a nylon carpet fiber. So, we can tell from the IR spectrum of the fiber that we measured, in red, and a library spectrum of nylon, in black, underneath it, that these spectra are quite similar. One would be hard-pressed to tell me anything specific about fillers or coatings from this type of spectrum. How can we learn more information about the fiber? Let’s take it to the instrument.
When we take the XPS spectrum of this fiber, we find that we get the elements that we expect to see in nylon: carbon, oxygen and nitrogen. However, we also notice something we did not necessarily expect to see: surface fluorine. And, it’s quite a strong signal, too. So, we have fluoride on the surface, probably from a surface treatment to prevent water beading and to prevent stains.
Going back to the infrared spectrum, I’ve highlighted in blue those regions of the spectrum where one might find the carbon-fluorine bond peaks. As we can see in nylon, there’s already a lot of peaks there. So, this would be a more complex way to try to look for fluorine on our nylon samples. However, in the XPS the fluorine stands out by itself.


Now you’ve seen how with XPS we can get some interesting information about a sample from the top ten atoms or so on a number of commonly found items. I spoke to you a little previously about ion beam depth profiling, from here on we’re going to discuss what value ion beam depth profiling brings to XPS.
The NEXSA that we use at McCrone Associates for performing XPS analyses is equipped with a dual mode ion source called the MAGCIS, the monatomic and gas cluster ion source. A source like this offers a lot of versatility for depth profiling. Its monotomic mode, in which a single ion is impinged into the surface at 500eV to 4keV, allows for deep surface penetration, ejecting a large number of electrons with each ion that hits, so, it has a high etch rate. However, an ion at this energy can cause damage to surface chemistry. Chemistry usually occurs in the 0 the 3 volts range. When you’re impinging with 200 volts to 4,000 volt ions you can affect this chemistry, much of which we are trying to find with XPS. However, for metallic materials, or glass, or ceramics, or other hard materials, this monatomic source is a good way to do depth profiling.
On the other hand, if you have soft material, such as polymers, the cluster ion source is the better way to go. It delivers a large cluster containing 75-2,000 atoms to the surface, which begins to break apart upon impact, which you see in the cartoon. You have low energy per atom, down to a volt, which means you do little damage to the surface chemistry. It is a lower etch rate, for softer materials a lower etch rate is usually not a problem. This large cluster is best for etching organic material or for just giving a gentle cleaning to an inorganic material or a hard metal prior to doing the analysis of interest. Otherwise, for many materials, you wind up looking at an adventitious carbon layer that you may not have much actual interest in. Now that we have discussed the dual mode ion source, we can show a few examples of how it might be helpful in analyzing our samples.
Let’s go back to the galvanized plates that I showed you previously. I showed you that just from the surface, while they look very similar in an SEM/EDS analysis, we find that the top few layers of atoms can be, in fact, quite different between the old plate and the new. We’re going to use the atomic source high-energy, high-penetration depth, and depth profile for a while on this galvanized plate. We can find that through the depth of the plate that their surface chemistries could not be more different.
We find that while both of them had an adventitious carbon layer, which you can see in the dark blue, and while the old plate has, as you would expect, a zinc layer, as shown in orange, and an oxygen layer, as shown in magenta, showing that it is an oxidized zinc layer on the outside of that plate. That newer plate is passivated. It has a different surface chemistry, which affects its appearance and, probably, over time, will affect how fast it corrodes compared to my older plate. We can see that the surface oxide is quite high, but as we penetrate into the depth, the oxygen atomic percentage compared to the other atoms goes down considerably, in purple.
We can see that at the very surface we don’t have a great deal of zinc, and that it starts to grow in after we’ve etched for 50 seconds or so. We note that it has a considerable amount of phosphorus within the first 150 seconds of etching time. We can identify that there is also some chrome in that first 150 seconds, or chromium, rather. And, we can note that after the chromium and phosphorus have been sputtered off, a silicon component starts to grow in as we continue to depth profile; silicon that was not observed in the old plate, phosphorus that was not observed at the surface of the old plate in this concentration. Through XPS we were able to determine the differences between these two plates.
Now that we’ve seen how XPS and depth profiling work on a conducting metal sample, the galvanized steel plate, let’s see if it works as well for depth profiling on a non-conducting sample. In this case, I have a piece of glass that was taken from the broken screen of a handheld device like many of the tablets and cell phones that people carry today. The monatomic ion source was used again for etching, and the flood gun was turned on, the electron flood gun, in order to prevent surface charging. This spectrum in red is the spectrum that was acquired before etching began. The overlaid spectrum in green was acquired 190 seconds into the experiment. As we can see, the surface of this glass has a considerable fluorine peak, so not terribly surprising that a fluoride coated glass would be desirable for preventing staining of the glass or other damage from water, and to make it easier to clean. Meanwhile, as we’ve depth profiled in, we see a few peaks growing in, notably, a magnesium peak as shown over there at roughly 1300 electron volts. There are a lot of ways we can visualize this data. I’ve shown you just two spectra overlaid from two different parts of the depth profile experiment, but we can show you a 3-D plot for the extent of the experiment that shows the surveys and the intensity of the peaks with respect to etch time. And you can see, for example, the fluorine line in that first one is a little spike that disappears rapidly as we etch. However, we can expand any part of this survey spectrum and monitor the change in any peak over time. For example, here we see the magnesium intensity slowly growing in as we continue to etch the surface.
Ion sputtering can also be used to learn things about surface treatment of plastic. Here is an example of that high density polyethylene again, the milk jug plastic that we placed in a plasma cleaner in order to oxidize the surface. You can see the strong peak for the carbon-carbon and carbon-hydrogen bond, and at higher energy on the oxidized side, we see that we have a small shoulder where the carbon-oxygen and the carbon double-bond oxygen peak is. For this sputtering experiment we will use the cluster ion source rather than the high-energy single ion source, and find that after sputtering for a period of time, going from the red trace for the original spectrum to the green trace for the spectrum following sputtering, that the carbon-carbon bond peak has increased in intensity, while the carbon-oxygen bond shoulder has decreased in intensity. To confirm that we’re removing oxygen from the surface and changing the chemistry, we can also go over to the oxygen line and see that it is decreasing in intensity as we sputter. This measurement was also performed with a flood gun on in order to prevent surface charging. As you can see, it works quite well.
Finally, the coated nylon fiber that we saw previously. Only a single round of sputtering under the cluster source completely eliminates the fluorine peak from the spectrum, leaving you with carbon, oxygen and nitrogen, as would be expected from a nylon sample.

In conclusion, the outermost atomic layers on the surface governs numerous materials’ properties. X-ray photoelectron spectroscopy takes advantage of the nanometer short electron path links in solids to provide information specific to those first few atomic layers. Atomic identification, oxidation state and bonding information all can be derived from those outermost atomic layers. With ion beam depth profiling a more complete understanding of the properties of the solid can be realized, including the thickness of passivation layers and their chemistries.
I’d like to thank you again for joining me today for this webinar on XPS. I’d also like to thank ThermoFisher Scientific for providing some of the graphics that were used in this webinar, and I’d also like to thank Dr. Sandra Koch for providing some of the spectra and assisting me with some of the samples.
CZ: Okay, thanks Doug for a great presentation and thanks again for everybody for attending today’s webinar. If you have any questions, please go ahead and type them into the questions field.
As we were rolling along here with Doug’s webinar, a couple of questions did come in. The first one is from Amy: “Can the etch times and these depth profiles be converted into nanometers?”
DM: The etch times and the depth profiles can, in fact, be converted to nanometers. It just takes a bit more work to do so. Essentially, you need to start out with a standard material and depth profile that for a specific amount of time through an aperture, and then measure the depth of that hole by some other means, a profilometer or another microscope. Because the etch rates of every material is going to be different, depending upon the etching conditions, this is really the only way to do that conversion, but it is possible.
CZ: Alright our next question is from Gloria, she wants to know: “Can molecular species be identified using XPS?”
DM: Generally speaking, molecular species really cannot be identified specifically using XPS. As you can see with the polymers, one can identify the type of bond, how oxidized and how reduced it is. In certain cases, the position of a peak can be somewhat characteristic, in very extreme cases, for a given molecule. But this is not the usual situation, and in most cases this technique is best for identifying what atoms are at the surface and what their general chemical environment is.
CZ: Okay, Doug. I think that’ll do it for the questions. I just want to make a quick note here to everyone that we’re planning a new series where we share tips and ideas to improve workflow and analysis. If you, perhaps, are a past student and would like to share something you learned in one of our classes, or if, as an analyst, you have found a microscopy hack, so to speak, and you are willing to share that with us and with our community here at one of our webinars, please send these ideas to Chris, there’s her contact information on the screen (webinars@mccrone.com), and we’ll include these ideas in an upcoming session. I would like to thank everybody again for attending today’s webinar and we hope to see you soon for another McCrone group webinar. Thank you.
Comments
add comment