Sand Gallery Update and Taking a Closer Look at Oil Sands

Listed below is how teachers from around the country have been using the free sand sample resources from the Microscopy Society of America’s (MSA) Sandbox. You can check out our previous Sand Gallery posts including Kinetic sand, beach volleyball sand, and others on Modern Microscopy by searching the word “sand”.
From Langdon, South Dakota
A teacher and director of a daycare center is having children look at different kinds of sand at low magnification. The students range in ages from three to five. The children use a magnifying glass and are encouraged to touch and feel the texture of each sand. They were willing to take whatever sands we had available. We decided to include some samples from the Sandbox collection that had not been photographed yet, and we also made some really cool sand study cards for the students to use. For more information on creating sand study cards, check out this article.





From Carnegie, Pennsylvania
A big thank you to Scott Donnelly and his class for donating sand to the Sandbox. Scott teaches Science Technology Engineering and Math (STEM), and Social Studies at Carnegie Elementary in Carnegie PA. He requested sand samples last year to use in his classroom, and as a thank you for the samples, some of his students collected sands from their vacations and sent them to the Sandbox program. How cool is that! This kind reciprocation is exactly how this resource continues to be replenished and made available as a free, ongoing program to science teachers.

In appreciation of his students’ generous and thoughtful contribution, we are making some sand study cards for his class; the actual sand sample will be attached, and a map from where the sample was collected, along with the name of its location, will be printed on each card.


Taking a Closer Look at Oil Sands
Microscopy courses at Hooke College of Applied Sciences, a member of The McCrone Group, are typically attended by a wide range of industry professionals. Such a diverse classroom environment gives our instructors the opportunity to hear about a variety of unique analytical applications and to take a look at interesting samples. This happens in a very literal sense, since we encourage students to bring samples from their labs in order to discuss them during class.
We recently had a couple of students from a major oil sands producer attend our Polarized Light Microscopy course. They brought with them a sample known in the industry as froth: an intermediate byproduct from the oils sands refining process. They also provided us with a cleaned oil sand sample to share with our science teachers as part of our Sand Gallery update.

Froth from oil sands production is the result of an initial gravity separation process where coarse solids, primarily sand, are removed from the recovered bitumen. At their laboratory, it is common to evaluate froth samples microscopically and take measurements of the water droplets and fine solids content that still remain in the bitumen. The froth is then treated with a solvent in an effort to reduce its viscosity and allow for the removal of residual water content and additional fine solids.
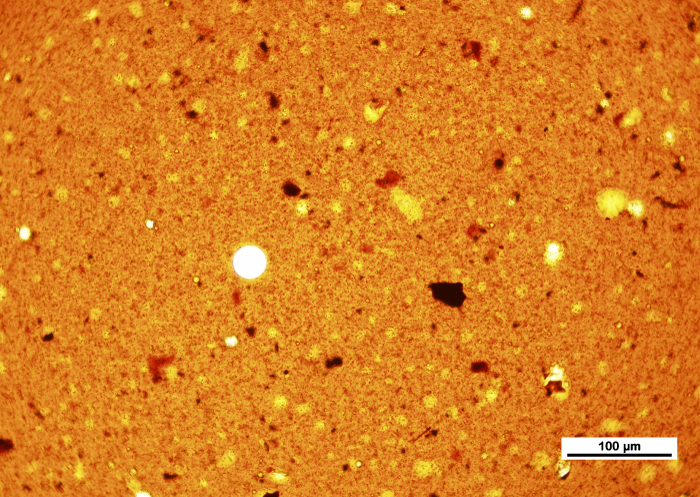
While the students explored their froth sample during class time, a number of great questions came up regarding the types of objectives one should use when looking at this highly viscous liquid sample. As mentioned above, the evaluation of froth involves the measurement of water content, which is in the form of droplets trapped within the bitumen. In their case, they used a variety of magnifications at their lab, and were considering replacing some of their microscope objectives. Students were weighing the trade-offs when it came to using traditional oil immersion objectives versus “high-dry” objectives. Their concern was in using oil immersion objectives, would the physical act of the oil immersion objective coming into contact with the coverslip influence the size of the water droplets? If it did, it could throw off the calculation used to determine the amount of solvent required to drive off the remaining water content contained in the froth. As with any microscopical analysis, having the proper objectives within a useful magnification range is essential to gathering meaningful results.
The students also provided this clean sample of sand recovered from the gravity separation process. Notice how most of the sand grains are smooth and rounded, similar to what you might find when looking at a beach sand.

If you have been using the MSA Sandbox samples in your classroom, let us know. We would love to tell your story to our readers. Remember, to receive Sandbox samples for an activity in your classroom please fill out a request on the MSA website.
Comments
add comment