Why is Particle Analysis So Effective?
Contaminants are materials that are not intended to be included in a manufactured product. These materials can often cause a product to function in an undesirable way, and a very small quantity can have a huge impact on taste, odor, physical function, tactile feel, or worse—if it is a toxic substance.
In traditional chemistry, contaminants or impurities are substances that occur in very small proportions; from a mass perspective, material that occurs at proportions much smaller than one weight percent. For example, some substances can make a product unusable in concentrations as small as a few hundred parts per billion; if this occurs and is homogeneously distributed in the mixture, it can be very analytically challenging to find an instrumental method capable of measuring the contaminant at all, to say nothing of doing it with precision (reproducibly) or with accuracy (the actual concentration). Bulk analytical methods such as inductively coupled plasma mass spectrometry can achieve both high precision and accuracy for some materials, however, the actual task can get difficult quickly.
For situations where the contaminants are not distributed uniformly in the product, a bulk analysis method is not reasonable for the task. Fortunately, in these instances, the contaminants tend to occur as heterogeneities or particles: discrete highly-concentrated accumulations of the contaminant. Optical microscopy and microscopy-based analysis methods provide the resolution to the concentration dilemma because they can be used to identify only the contaminant material, without a dilution effect of the rest of the product material.
-

A tablet with a brown stain, mounted on an aluminum disk with a conductive carbon adhesive for scanning electron microscopy. -
The electron micrograph of the brown material; the stain doesn’t show up well against the background of the tablet, but you can see the stain is composed of particulate matter.

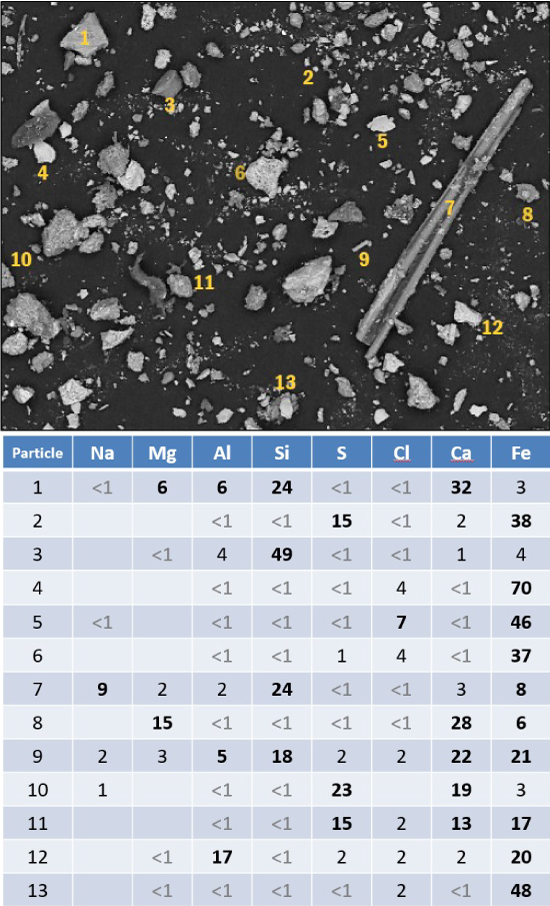
Particles are the tiny traces of evidence of undesired or unexpected processes. Particle analysis allows us to identify contaminant materials which helps our clients trace production processes back to their source so they may be eliminated. Contact us to discuss your contaminant concerns.
Comments
add comment