Uses for Flexible Collodion in the Analysis of Small Particles
Flexible collodion is a name given to a solution of 4 g of nitrocellulose in 100 mL of a mixture of 25 mL of ethanol and 75 mL of ether, with 2% camphor and 3% castor oil (by wt.); the camphor and castor oil impart the flexibility. It can be found in ½ liter bottles in most chemical supply catalogs. Flexible collodion solution should not be confused with plain collodion solution; collodion solution does not contain the camphor and castor oil, and is not flexible. This formulation was first used as a glue (Greek Kolla glue).
About 0.5 mL of the lightly-viscous solution spread over 2/3 of a microscope slide will form a 25 µm thick film of nitrocellulose with a trace of castor oil, when the solvents evaporate. This film is referred to in this article as collodion film. In a well-ventilated area, a number of these slides should be made at one time, and stored in petri dishes.
Collodion film is a very popular sample preparation medium. It is conveniently available on a microscope slide and can be reconstituted instantly (made lightly viscous) with a single drop of amyl acetate. Small drops of the reconstituted sample on the end of a tungsten needle are used for isolating, characterizing and preparing small particles for further analysis. The reconstituted sample will remain lightly viscous for up to 15 minutes. The slide can be reused many times and stored indefinitely (see Figure 1).
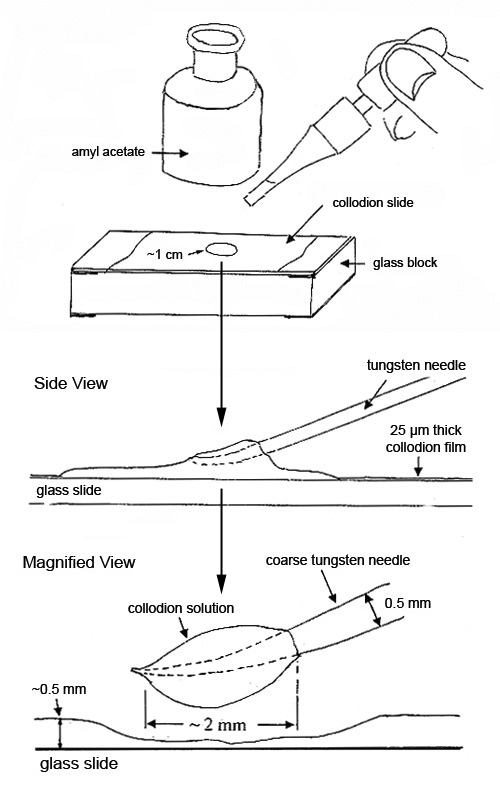
If the particles or substrate dissolve in amyl acetate, the following options are available: drops of the flexible collodion solution as received, which contain ether and ethanol, can be used instead.
If ether reacts with the substrate or particles on which flexible collodion solution is to be used, then the ether can be replaced with additional ethanol as follows:
- A 1:1 flexible collodion solution and ethanol are heated in a water bath at about 40°C with constant stirring until no more bubbles of ether are coming off. The resulting solution should be used within the next few hours because the solution does not keep.
When larger quantities of nitrocellulose in amyl acetate solution than those picked up with a tungsten needle from a microscope slide are required, then the flexible collodion solution in the pint bottle is treated as follows:
- In a well-ventilated area, 6 mL of the collodion solution and 3 mL of amyl acetate are poured into a 15 mL test tube. The tube is placed in a water bath at about 40°C and stirred constantly with a disposable pipette. It will bubble vigorously for 1 to 2 minutes or until almost all of the ether and some of the ethanol have evaporated. This solution can be stored in Teflon™ stoppered vials for up to two years.
Two examples of how the reconstituted solution is used follow.
Removing Contaminants—Without Altering Their Positions—From Soft or Rough Surfaces or From Pits or Craters Where the Tungsten Needle Cannot Reach
This extraction replication technique is especially useful when the surface is rough and soft at the same time. The collodion film will separate the particles lightly held in crevices without removing any of the substrate as follows:
Step 1. Two drops of the collodion solution are picked up on a tungsten needle and spread over about 10-20 mm2 of the contaminated surface. More drops may be used to cover a larger area. A third drop may be necessary to obtain a final film thickness of about 20 µm. Thinner films are fragile and harder to remove. The solvent should evaporate in 5-15 minutes at room temperature. The preparation can be dried faster in an oven at about 60-80°C (see Figure 2).
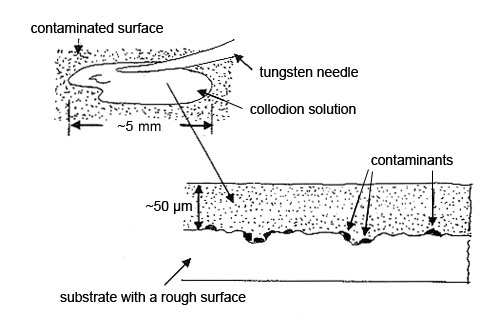
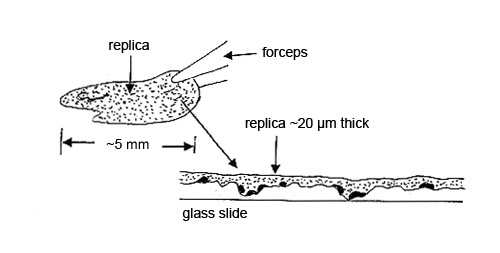
Step 2. The dry collodion film with the particles is pulled off the substrate and placed, particle side down, on a glass slide for analysis by polarized light microscopy. The contamination particles may be hard to characterize if, for example, the substrate was a roughly machined part. The replica of the machining marks may interfere with the microscopical characterization (see Figure 3).
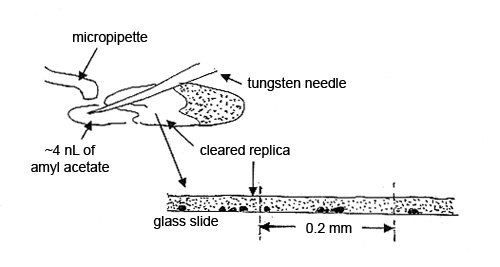
Step 3. A 3-5 nL drop of amyl acetate, delivered from a micropipette, is brought in with a tungsten needle and allowed to flow halfway under the collodion film. This area of film is referred to as the “cleared replica.” The particles in the cleared portion of the 20 µm film of collodion are clearly visible with a polarized light microscope. The film acts like a refractive index liquid with nD=1.51. The other half of the film is used for comparison (see Figure 4).
Analysis of the Contaminants by SEM/EDS
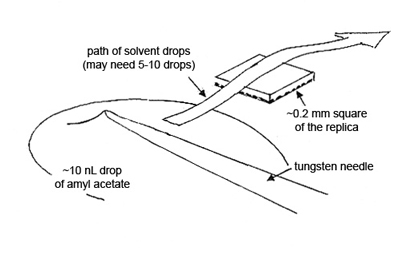
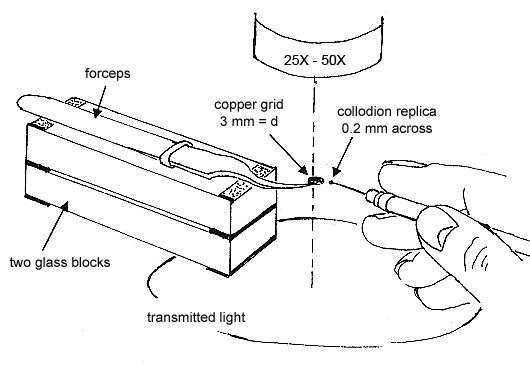
To obtain the elemental compositions of the contaminants, cut out a square of the replica, measuring ~0.2 mm, transfer it to a polished carbon or beryllium substrate, and dissolve the collodion by quickly passing small drops of amyl acetate over the square. Allow each drop to dry before passing another drop over the replica (see Figure 5).
Isolating Contaminants in Glass
There are many products that depend on various types of glasses to be particle-free down to the submicrometer level, e.g., optical fibers and glass lenses. Isolating micrometer to submicrometer contaminants in glasses is challenging; however, collodion film and a custom-made diamond scribe can make the task much easier. Isolating a contaminant in glass is illustrated below. Isolating a contaminant from an optical fiber will be illustrated at a later date.
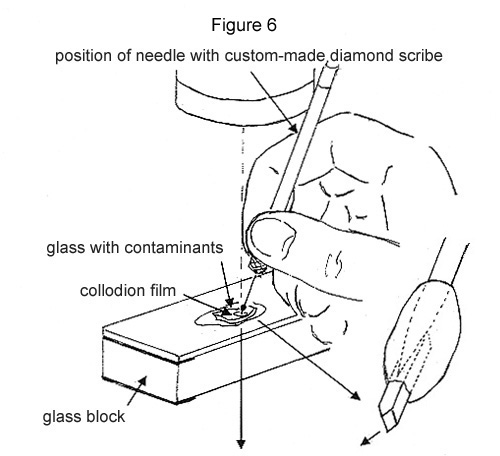
Step 1. A contaminant is located no farther than 100 µm below one of the glass surfaces. If one is not found, then the glass has to be fractured, exposing fresh surface, until one just below the surface has been located. Its location is carefully marked with the custom-made diamond scribe. One or two drops of the reconstituted collodion solution are put on top, allowed to dry, and form ~10 µm film.
Step 2. The glass directly above the contaminant is crushed with the custom-made diamond as illustrated. The collodion film will keep the fragments from scattering (see Figure 6).
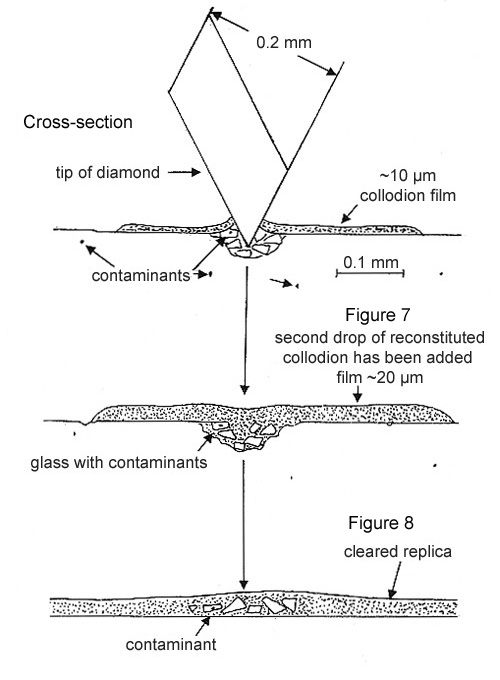
Step 3. The crushed fragments and the cut collodion film are fused together with a 10 nL drop of amyl acetate, followed by a drop of the reconstituted collodion solution. After the film dries, it is peeled off the glass surface and placed on a microscope slide (see Figure 7 above).
Step 4. The film is “cleared” with a drop of amyl acetate and then examined with PLM at 500X magnification and strong illumination. Frequently, the contaminant has a much higher refractive index than the glass and is easy to relocate (see Figure 8 above).
If the contaminant is not found in or among the glass fragments, it most likely has not been dislodged (see Figure 6). Repeat until the contaminant is located (see Figure 8). This may occur when the contaminant is >50 µm below the surface of the glass.
Step 5. The glass fragment with the contaminant is separated from the rest as follows: the collodion film can be made viscous with amyl acetate and the fragment can be picked out with a tungsten needle. Or the glass fragments can be spread over a larger area in the viscous collodion and the fragment with the contaminant can be cut out when the amyl acetate evaporates.
Step 6. If the contaminant in the glass fragment is not exposed sufficiently to obtain elemental and compound data, it is prepared for crushing on a custom made diamond press as follows: the ~0.2 mm replica with the <50 µm glass fragment is placed on the bottom diamond and most of the collodion is dissolved.
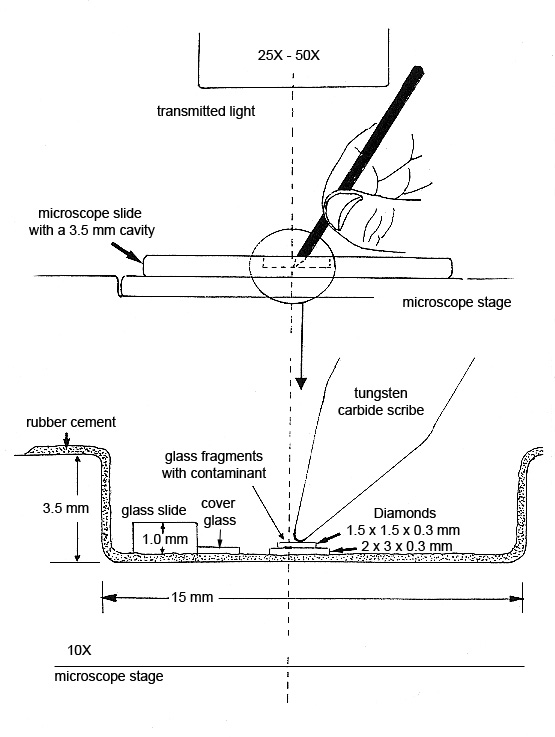
Step 7. The diamond cell is made from a deep well slide that has been coated with rubber cement*. The sample to be pressed is placed between two diamond plates resting on the bottom of the well. See diagram below. More pressure can be applied by hand with a tungsten carbide scribe than can be applied with a commercially available diamond cell.
The microscopist can observe the fragment and the collodion film spreading between the two diamond surfaces. The thickness of the preparation is estimated from the interference colors that form between the two diamond surfaces when they are being pressed. Films ~300 nm (orange) are easy to obtain and are suitable for analysis on the analytical electron microscope (AEM) (see Figure 9).
Step 8. The pressed sample can be removed from the diamond surface using the extraction replication technique described above in the section Isolating Contaminants in Glass, Steps 1 and 2. Approximately 0.2 mm square of the replica that contains the pressed sample is placed either on a polished substrate for elemental data on the SEM/EDS or on a carbon-coated copper grid for compound identification by electron diffraction on the AEM. The collodion is dissolved from the two preparations as illustrated in Figure 5. Part of the AEM preparation is illustrated in Figure 10.
This procedure works well for submicrometer-to-micrometer size inclusions in glass matrices.
* A rubber cement coating keeps the lower diamond in place and prevents it from damaging the bottom of the well slide. There is also less chance of losing the upper diamond when the well slide surfaces are coated with rubber cement.
Comments
add comment