Flow Imaging Microscopy: Identifying Subvisible Particulate in Biotherapies (Proteinaceous or Cellular-based)
The acquisition of a Yokogawa Fluid Imaging Technologies FlowCam 8000 by McCrone Associates (MA) provides a new tool to assist clients with determining if their product has a subvisible particulate problem. Flow imaging microscopy (FIM) is complementary to our existing arsenal of microscopy-based techniques configured for the identification of unexpected and/or foreign particulate in product materials. This new capability allows for the visualization of particles suspended in fluids, which is critical for a successful analysis and identification. Biotherapies, whether protein- or cellular-based, have a significant barrier to assessing whether they contain subvisible contaminants or not. That barrier is the product matrix.
USP <788> outlines the testing of injection materials by two methods. Method 1 prescribes light obscuration for enumeration of particles, while Method 2 prescribes filtration and manual optical microscopy for particle enumeration. Each method has different advantages and disadvantages. Method 1 is automated and objective but lacks contextual information about the particles analyzed. While Method 2 utilizes the good judgement of trained microscopists to determine which particles meet the USP criteria for particulate matter, but particle enumeration and measurement is more subjective. FIM combines the advantages of both methods to deliver a comprehensive and objective representation of the particulate matter present in solution. The FIM-captured images demonstrate whether particles of a particular size are extraneous or not. Cellular media, protein wisps, and gas or silicone bubbles are easily distinguished from other extraneous subvisible particles.
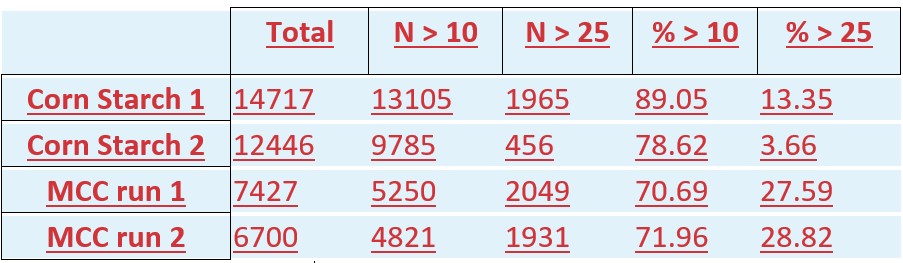
MA has traditionally not conducted USP <788> because we specialize in particle identification rather than enumeration. Acquisition of the FlowCam is exciting for MA because it provides supplementary information to our current approach, and it allows us to prescreen challenging samples. Particularly for proteinaceous and cellular-based biotherapies, FIM offers significant advantages over light obscuration and filtration in two major ways: first, FIM produces images of the particles (these can be interpreted using automated software), and second, the drug product can be analyzed in solution without suffering significant chemical state changes that occur with filtration (filtration is sometimes not possible with proteinaceous and cellular biotherapies).

McCrone Associates uses FIM to first evaluate biotherapy injections for foreign particle types. If foreign contaminants are found we have accurate enumeration and size measurements, and we can subsequently perform particle identification using such methods as FTIR spectroscopy and SEM/EDS. The FIM images of these other particle types are used to guide isolation of representatives for analysis.
Contact us to learn more about how we can collaboratively assist with your subvisible particle identification needs using flow imaging microscopy.
Further details about using FIM to analyze Subvisible Particles in Protein and Cell & Gene Therapies can be found on Yokogawa Fluid Imaging Technologies’ blog page.
Comments
add comment