Using XPS to Find Evidence of Ammonia Corrosion of Copper
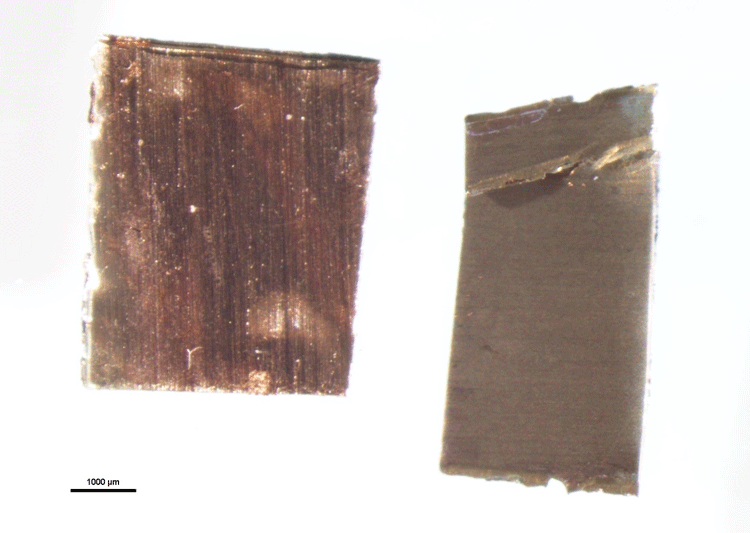
At McCrone Associates, we are often asked to assist our clients with determining the root cause of metal corrosion. For corrosion of brass, bronze, or other copper-based alloys, a possible culprit may be exposure of the metal to ammonia. Ammonia and water can react with copper-bearing surfaces to form a brittle copper-ammonium complex. This reaction initially dulls the surface finish of the metal (Figure 1), and with continued exposure can facilitate ammonia-induced stress corrosion cracking.
Since visual examination of a given specimen may be inadequate to identify the corrosive agent, spectroscopic methods that can classify chemical species are valuable tools for identification of root cause, enabling the client to more specifically target the source of a problem. Here, we demonstrate how one of our tools, X-ray photoelectron spectroscopy (XPS), can be used to confirm ammonia-induced corrosion of a copper surface. This non-destructive quantitative method can be used in place of the qualitative “hanging drop” test, an older, destructive laboratory test for ammonium that depends upon observing crystal formation in a chloroplatinic acid solution.
XPS can be beneficial to a corrosion analyst in two ways. First, it’s an electron spectroscopy for chemical analysis (earning it the acronym “ESCA” when the method was first adopted) with good energy resolution — approximately 1 eV for many peaks. This allows the analyst to not only identify the elements by their peak position, but also frequently to determine the chemical state, including bonding and oxidation state, specific to those elements. In some cases, an element can be found in multiple chemical states; for example, multiple states are commonly observed in organic compounds, as well as in oxidized passivation layers found on metals. Second, it’s information depth is quite shallow — on the order of a few nanometers deep. Since most corrosion reactions are initiated with the outermost atoms of a specimen, this excellent depth resolution improves the likelihood of measuring products of the corrosion reaction most relevant to the analysis, minimizing signal from relatively unaffected and irrelevant bulk material.
Identifying potential sources of corrosion may not be as simple as analyzing the composition of a corroded part. Often, knowing what evidence to look for beyond composition, and knowing how to find it, are critically important to the investigation, particularly if XPS is a key analytical tool for solving a given problem. Ammonia corrosion of copper is a prime example of this. Figure 2 shows XPS survey spectra of the untreated and exposed copper foils shown in Figure 1, along with the compositional analysis.

A casual glance at the numbers shows that while the exposed copper surface contains more oxygen than the untreated surface, they also indicate the surface nitrogen concentration is actually lowered following ammonia vapor exposure! This paradoxical result may lead one to the incorrect interpretation that ammonia cannot have been the culprit. The key to avoiding that mistake is to acquire spectra at higher energy resolution, thereby monitoring the subtle details of peak position that reveal not only what is at the surface, but also what is its chemical state. It is the chemical state information that allows the analyst to better explain how a given surface composition may have formed.
In order to determine whether or not chemical state information can be used to characterize the corrosion mechanism, region-of-interest (ROI) XPS spectra were acquired of each element on the copper surfaces. These spectra are useful for confirming that the copper was indeed oxidized by exposure to the ammonia; the evidence is the appearance of strong Cu(II) satellite peaks in the Cu 2p spectrum of the exposed specimen when compared to that of the untreated specimen (Figure 3). However, the key to confirming that ammonia caused this oxidation can be found in the nitrogen spectral region (Figure 4).

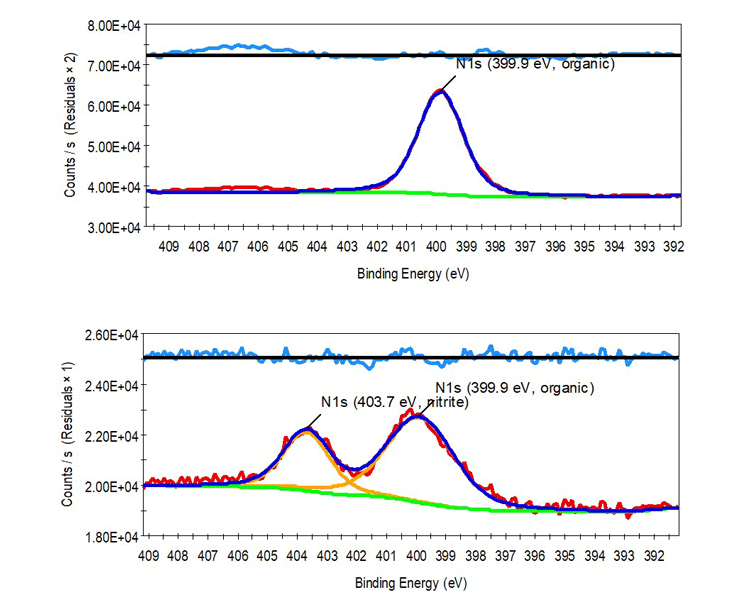
Here, one can observe that both the exposed and the untreated copper specimens exhibit a single N 1s peak at 399.9 eV, attributable to nitrogen substituents of adventitious organic molecules commonly observed on air-exposed surfaces. However, the ammonia-exposed copper exhibits a second N 1s peak at 403.7 eV, indicating presence of the nitrite (NO2-) ion. Nitrite forms when copper hydroxide, typically found in small amounts on air-exposed copper surfaces, oxidizes the tetraamminecopper complex produced upon ammonia exposure. This nitrite peak is clear evidence that the copper was likely exposed to ammonium hydroxide vapor.
The analysis of tarnished copper for evidence of ammonia-induced corrosion is one example of how XPS is an integral component of McCrone Associates’ multifaceted investigational analytical approach to solving corrosion problems.
Comments
add comment